3474
Dynamic MRI of Nitroxide Radical for TEMPOL kinetics and Redox State Assessment in Porcine Aortic Wall1Chair of Cellular and Molecular Imaging, Comprehensive Heart Failure Center, University Hospital Wuerzburg, Wuerzburg, Germany, 2Translational Center Regenerative Therapies, Fraunhofer Institute for Silicate Research, Wuerzburg, Wuerzburg, Germany, 3Institute of Anatomy and Cell Biology, University Wuerzburg, Wuerzburg, Germany, 4Institute for Experimental Biomedicine II, University Hospital Wuerzburg, Wuerzburg, Germany
Synopsis
Reactive oxygen species (ROS) plays a key role in vascular disease. The physiological mechanisms regulating vascular local oxidative stress (LOS) are not completely understood. Nitroxide radicals like TEMPOL have been used in basic science NMR studies of ROS and LOS. Therefore, we developed a technique for dynamic imaging of nitroxide radicals to assess their kinetics and redox state of tissue.
Introduction
Reactive oxygen species (ROS) play a key role in vascular diseases. The physiological mechanisms regulating vascular local oxidative stress (LOS) are not completely understood. Essential factors are oscillatory shear on the endothelial cell surface, and transduction to the rest of the vascular wall. The LOS in the extracellular matrix appears to be of importance as potential trigger of plaque rupture. Therefore, there is a critical need for the non-invasive methods of recognizing the vascular LOS at early stages. Nitroxides (stable nitroxyl radicals) can react with ROS with conversion to diamagnetic species and, thus, losing their paramagnetism1. Because of these properties nitroxides are used as NMR contrast agents sensitive to redox environment. Recently, the potential of long-lived heparin-nitroxide-conjugates contrast agents for labeling the vessel wall has been demonstrated both ex-vivo and in-vivo2,3. The redox sensitivity of nitroxides significantly varies, depending on local environment. For understanding the connection of pathophysiological changes of vascular tissue on the redox state it is important to observe the kinetics of nitroxide conversion at high spatial resolution. In this paper we demonstrate a series of proof-of-principle experiments to establish MRI technique for monitoring the kinetics of stable radicals and the redox state of nitroxides inside the porcine aorta vessel wall.Materials and Methods:
Excised porcine aorta was kept in isotonic (0.9% NaCl) solution at room temperature. Aortic samples were prepared as a 5mm thickness rings immediately before measurements performed at T=2/24/48 hours after excision. 10-30mM TEMPOL solutions were used. Probed and control aortic rings were simultaneously incubated at 37ºC for 3 min in TEMPOL and in isotonic solutions respectively. Samples were placed in scanner and TEMPOL kinetic was monitored for 50-60 min by continuous series of MR-scans. Afterwards, the probed ring was exposed for 5 min to 10-20mM ascorbic acid solution (37ºC) used to mimic the strong ROS outcome and MR-scanning were proceed for 30-35 min.
All measurements were performed using small animal PharmaScan 7T scanner (Bruker BioSpin, Ettlingen, Germany) using a dual-channel TX/RX cryoprobe. The pulse sequences parameters have been preliminary adjusted to achieve optimal SNR/CNR, temporal and spatial resolution for the visualization of TEMPOL kinetic inside the aortic wall. The T1-weighted GRE and turbo-SE (RARE) pulse sequences were used. Sequences parameters were as following: GRE: TE/TR/FA=2.5ms/30-80ms/75-90º, SE: TE/TR/FA=7-17ms/80-250ms/90º, in-plane pixel size=130-160µm, slice thickness=0.3-0.75mm, number of slices=2-5. The number of averages was adjusted to provide temporal resolution of 30-180 sec/image depending on number of phase encodings and RARE-factor.
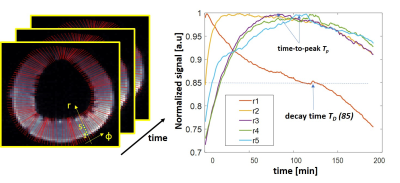
Basing on the resulted time series of images the local T1-contrast variation was evaluated reflecting the local kinetic of the TEMPOL redox state. Calculated parameters were time-to-peak Tp value and decay times TD(x) to specific level x% characterizing both TEMPOL uptake and reduction under endogenous ROS. For reduction of TEMPOL by ascorbic acid the additional exponential fit decay time τD was calculated to determine the dynamic range weighted decay rate as RDR=(Smax-Smin)/τD.
Results and Discussion
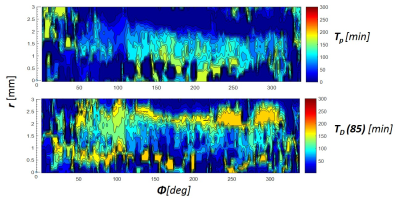
Figure 1 demonstrates exemplary images acquired after incubation of probed ring in 10 and 30ml of TEMPOL solution at different time points and after incubation in ascorbic acid. The TEMPOL penetration inside the wall and reduction both by native ROS and ascorbic acid is measurable even at relatively low concentrations. Figure 2 and 3 shows spatial and temporal profiles reflecting the kinetic of TEMPOL penetration and conversion to diamagnetic of hydroxyamide inside the wall due to presence of endogenic ROS. The form of time-signal profiles in specific region depends on the distance from the surface of the wall and represents combination of TEMPOL uptake by tissue and its conversion due to presence of ROS. The time-to-peak time for uptake and decay time for conversion are mapped and shown on Figure 3. Figures 4 and 5 demonstrate the effect of the ascorbic acid on the TEMPOL redox state. The maps of signal decay reflect the penetration of ascorbic acid in tissue and conversion of TEMPOL to the diamagnetic state.Conclusion
We have demonstrated that TEMPOL redox kinetics in porcine vasculature can be effectively monitored by MRI in ex-vivo tissue specimens. Optimized sequence parameters provide necessary combination of SNR, temporal and spatial resolution to map the rate of TEMPOL conversion in tissue on pixel basis. This allows correlating the redox state changes with underlying morphology of the vessel wall layers (endothelial, intima, media, etc). The current study is the step for further kinetic experiments in vessel tissue placed in oxygenating organ chamber under physiological conditions and in small animals in-vivo.Acknowledgements
Financial support: German Ministry of Education and Research (BMBF), grant #01E1O1504.References
1. May et al. Ascorbic acid decreaseas oxidant stress in endothelial cells caused by the nitroxide tempol. Free Radical Research, February 2005; 39(2): 195-202.
2. Kleschyov et al. Heparinepolynitroxides: Synthesis and preliminary evaluation as cardiovascular EPR/MR imaging probes and extracellular space-targeted antioxidants; European Journal of Medicinal Chemistry 58 (2012) 265-271
3. Terekhov et al; Heparin-Polynitroxide Derivatives: First Application as Site Specific MRT Imaging Contrast Media for Vascular Wall, Proceedings of ISMRM, Melbourne, Australia 2012;
Figures
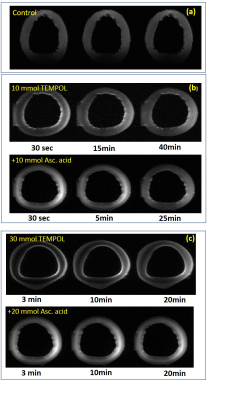
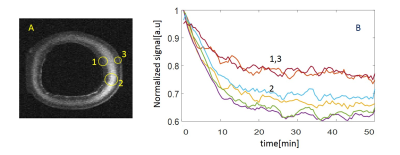
(a) Temporal Dynamic Range (Smax-Smin) projection image of aorta ring due to elimination of TEMPOL contrast enhancement after ascorbic acid treatment. The TEMPOL in surface zones is mostly eliminated already during treatment. Maximal variation of the signal is as expected in the central zone.
(b) Exemplary temporal profiles of the normalized signal in the zones marked 1-3 on the panel (a) demonstrates difference in both dynamic range and rate of signal decay in different zones.
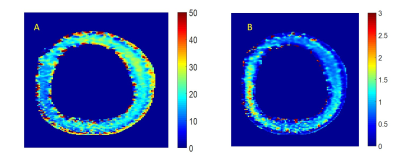
(a) Characteristic half decay time D(50) of the TEMPOL induced contrast in the aorta tissue during reduction by ascorbic acid.
(b) TEMPOL elimination decay rate calculated from exponential decay time τD and local dynamic range of the signal (see text). Tissue zone discrimination and local variations of TEMPOL elimination rate by ascorbic acid can be clearly observed with high spatial resolution.