3470
Imaging uptake of plasma macromolecules in the arterial wall1Department of Bioengineering, Imperial College London, London, United Kingdom, 2Biomedical Engineering, King's College London, London, United Kingdom
Synopsis
Elevated endothelial permeability is a precursor to atherosclerosis. Imaging macromolecule uptake in the artery wall can be used to detect and investigate early structural and functional endothelial dysfunction. Current techniques make use of destructive post-mortem analysis of tissue, limiting studies to animal models only. MR imaging of the transport of an albumin-binding contrast agent (Gadofosveset) could be used instead. We employed a mathematical model to differentiate between the bound and unbound fraction of the contrast agent thus making this method a promising non-invasive technique to measure permeability in humans for the first time.
Introduction
The focal distribution of atherosclerosis has been attributed to local haemodynamic influences on the endothelium, which are thought to increase endothelial permeability to circulating macromolecules [1]. Imaging macromolecule uptake by the artery wall can provide an insight into disease development, but existing methods are limited to animal models [2]. MR imaging of an albumin-binding contrast agent (Gadofosveset) holds promise for studying endothelial permeability in humans non-invasively; however, only 75% of the contrast agent is bound to albumin at the clinical dose, adversely affecting measurements [3,4]. The technique has also not been validated against existing methods.Methods
A mathematical model can generate a concentration map of CA bound to albumin within the artery wall [5]. This involves the acquisition of T1 and T2 maps of the vessel before and after administration of Gadofosveset (∆R1 and ∆R2), and determining the bound and unbound relaxivity, r1 and r2, in vitro [5]. Relaxivity was measured in PBS-based solutions and tissue-mimicking collagen gels at 21oC and 37oC at 3 T. The model was then applied to in vitro models of arterial permeability. Solutions of Gadofosveset, with and without albumin, were placed on collagen gels and allowed to diffuse into them for 30 mins. The study was repeated using CA covalently bound to albumin (Galbumin, BioPAL Inc.). The MRI technique was compared to confocal microscope imaging of collagen gels exposed to rhodamine-labelled albumin.
$$[CA_{bound}]=\frac{r_2^{free}\triangle R_1-r_1^{free}\triangle R_2}{r_1^{bound}r_2^{free}-r_2^{bound}r_1^{free}}$$
In vivo imaging was performed on male Sprague Dawley rats injected with Gadofosveset (0.03 mmol/kg, iv). Cross-sections of the brachiocephalic artery were imaged before and 30 mins after administration.
Results
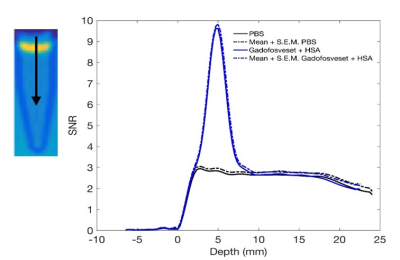
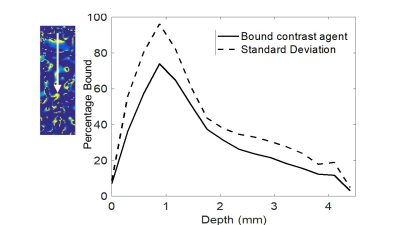
In vitro relaxivity of Gadofsoveset increased upon binding to albumin and was dependent on temperature and viscosity. Therefore, r1/r2 of collagen gels at 37oC should provide the best estimate of in vivo relaxivity. The uptake of Gadofosveset plus albumin by collagen gels was studied by plotting signal intensity from weighted images vs distance into the gel. Gadofosveset appeared to penetrate 5 mm into the gel (Figure 1), whereas bound CA penetrated 1 mm (Figure 2) and rhodamine albumin, detected by confocal microscopy, penetrated 200 µm.
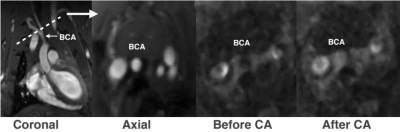
In vivo data appeared to have some albumin uptake in the weighted images of healthy rats but further work is required to ascertain this (Figure 3). The resolution was also sufficient to visualise the brachiocephalic artery.
Discussion
Relaxivity is dependent on environmental factors. In viscous solutions and lower temperatures the rotational correlation time increases, leading to an enhancement in relaxivity. The difference in measured penetration depth between unbound and bound CA (5 mm vs 1 mm, respectively) suggests that the model, when used with appropriate values of r1 and r2, is able to remove effects of unbound CA, which would be expected. The further discrepancy with the confocal microscopy result for covalently labelled rhodamine-albumin could be related to partial volume effects with MRI. Preliminary in vivo scans confirmed that scanner resolution is sufficient to visualise the brachiocephalic artery in rats.Conclusion
The model appears to be a promising method for removing effects of unbound CA and thus may provide a quantitative measure of albumin uptake into the artery wall. This could lead a non-invasive technique for investigations of the role of arterial wall permeability in the pathogenesis of atherosclerosis and to correlate wall shear stress with permeability in humans for the first time.Acknowledgements
This study was funded by the EPSRC, BHF and Imperial College Biological Imaging Centre. Thank you to Dr E.M. Rowland for image processing advice, Dr. B. Lavin Plaza and Dr T. Schneider for their MRI expertise.
References
[1] Staughton TJ, et al., Effect of altered flow on the pattern of permeability around rabbit aortic arches, Am J Physiol Heart Circ Physiol, 281: H53–H59, 2001.
[2] Bailey EL, et al., Mass Transport Properties of the Rabbit Aortic Wall, PLoS One, 10: 1–20, 2015.
[3] Phinikaridou A, et al., Noninvasive Magnetic Resonance Imaging Evaluation of Endothelial Permeability in Murine Atherosclerosis Using an Albumin-Binding Contrast Agent, Circulation, 126(6):707-19, 2012.
[4] Caravan P., et. al., The Interaction of MS-325 with Human Serum Albumin and Its Effects on Proton Relaxation Rates, J. Am. Chem. Soc., 124: 3152- 3162, 2002.
[5] Richardson OC, et al., Gadofosveset-Based Biomarker of Tissue Albumin Concentration: Technical Validation In Vitro and Feasibility In Vivo, Magn. Reson. Med, 73:244–253, 2015.
Figures