3469
PD-T2-Shuffled Volumetric ISotropic Turbo spin echo Acquisition (VISTA) for 3D Simultaneous Multi-contrast Intracranial Vessel Wall Imaging1Center for Biomedical Imaging Research, Department of Biomedical Engineering, Tsinghua University, Beijing, China, 2Philips Research North America, Cambridge, MA, United States, 3Vascular Imaging Lab, Department of Radiology, University of Washington, Seattle, WA, United States
Synopsis
The aim of this study was to develop a PD-T2-shuffled VISTA technique, which can provide co-registered 3D high multi-contrast intracranial vessel wall images with high scan efficiency. Phantom study and healthy volunteers study were performed to validate the feasibility of the proposed method. The results showed that middle cerebral artery vessel wall was clearly depicted on different weighed images of PD-T2-shuffled VISTA.
Introduction
Intracranial arterial stenosis, resulting from atherosclerosis, vasculitis, thrombosis and other reasons, is one of the major causes of ischemic stroke [1]. Recently, three-dimensional variable flip angle turbo spin echo (TSE) techniques, such as VISTA (Volumetric Isotropic TSE Acquisition; Philips Healthcare) [2], SPACE (Sampling Perfection with Application optimized Contrasts by using different flip angle Evolutions; Siemens Healthcare) [3] and CUBE (GE Healthcare) [4], emerged as the mainstream technique for intracranial vessel wall imaging (IVWI). Although multi-contrast IVWI showed its power in plaque vasculitis discrimination [5] and plaque composition detection [6], the application of multi-contrast IVWI is limited by its long scan time (>20 minutes [5]) and potential intra/inter-scan motion.
In this study, we aim to propose a PD-T2-shuffled VISTA technique, which can provide co-registered 3D high resolution multi-contrast intracranial vessel wall images with high scan efficiency.
Methods
Pulse sequence:
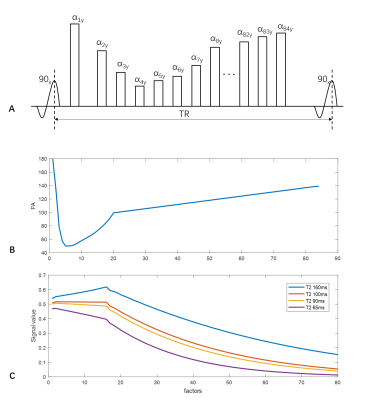
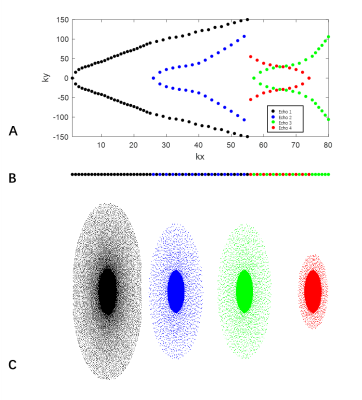
The VISTA sequence with variable refocusing flip angle was previously optimized for intracranial vessel wall imaging (Figure 1) [2]. To facilitate multiple contrast imaging, the profile order of proton density weighted VISTA sequence was rearranged (Figure 2a,b). In this work, an echo train was split into 4 segments to generated 4 separated K-spaces (KSP1, KSP2, KSP3, KSP4) with different echo times (TE1, TE2, TE3, TE4). Moreover, sparse sampling with CUSTOM [7] was used to speed up the acquisition. As image contrast was mainly determined by K-space center, center K-space lines of of KSP2, KSP3 and KSP4 were acquired more frequently than outer K-space lines. The acceleration factors of KSP1, KSP2, KSP3 and KSP4 were 3, 8, 8 and 12, respectively.
Image reconstruction:
KSP1 with TE1 was reconstructed directly with 3D SPIRiT [8]. A view-sharing technique was first applied to KSP2, KSP3 and KSP4 followed by 3D SPIRiT reconstruction.
MR Imaging:
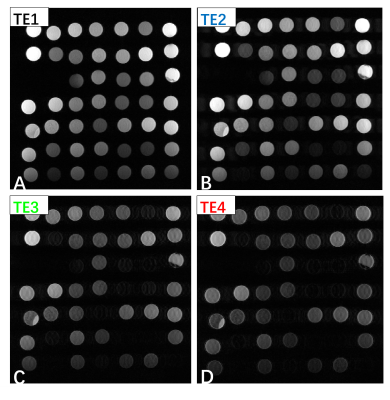
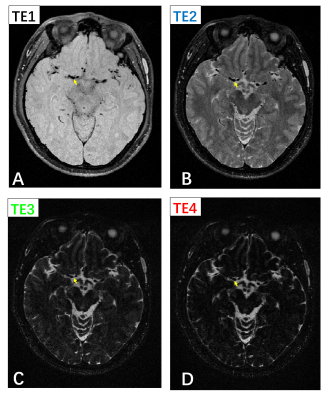
To validate the feasibility of PD-T2-shuffled VISTA, a set of tube phantoms with different T1 values and T2 values was scanned. Also, two healthy volunteers was scanned with the following parameters: TR 1800 ms, effective TE 30/137.5/245/352.5 ms, echo spacing 4.3 ms, echo train length 84, startup echoes 4, resolution 0.6*0.6 *0.6mm3, scan time 7min08s.
Results
Figure 3 shows the phantom study result. Signal change due to T2 decay can be observed in images with different TEs (Figure 3 A-D). Figure 4 shows one invivo result. The vessel wall of middle cerebral artery was clearly depicted in all images (yellow arrow). The contrast between white matter, gray matter and cerebral spinal fluid (CSF) varied between images with different TEs (Figure 4 A-D).Discussion and conclusion
This study demonstrated that PD-T2-shuffled VISTA is a feasible and promising technique for 3D simultaneous multi-contrast intracranial vessel wall imaging. The multiple weighted images could provide more information for plaque components detection and vasculopathy differentiation, which would be investigated in the future.Acknowledgements
No acknowledgement found.References
1. Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. 2006;1:158–159.
2. Qiao Y, Steinman DA, Qin Q, Etesami M, Schär M, Astor BC, et al. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 tesla. J Magn Reson Imaging 2011;34:22–30.
3. Xie Y, Yang Q, Fan Z, Li D. Improved black-blood imaging using DANTE- SPACE for combined carotid and intracranial vessel wall evaluation. J Cardiovasc Magn Reson 2015;17:O17.
4. Li ML, Xu YY, Hou B, Sun ZY, Zhou HL, Jin ZY, Feng F, Xu WH. High-resolution intracranial vessel wall imaging using 3D CUBE T1 weighted sequence. European journal of radiology. 2016 Apr 30;85(4):803-7.
5. Mossa-Basha M, Hwang WD, De Havenon A, Hippe D, Balu N, Becker KJ, Tirschwell DT, Hatsukami T, Anzai Y, Yuan C. Multicontrast high-resolution vessel wall magnetic resonance imaging and its value in differentiating intracranial vasculopathic processes. Stroke. 2015 Jun 1;46(6):1567-73.
6. Jiang Y, Zhu C, Peng W, Degnan AJ, Chen L, Wang X, Liu Q, Wang Y, Xiang Z, Teng Z, Saloner D. Ex-vivo imaging and plaque type classification of intracranial atherosclerotic plaque using high resolution MRI. Atherosclerosis. 2016 Jun 30;249:10-6.
7. Zhou Z, Chen S, Sun A, Li Y, Li, R, Yuan C. Optimized Parametric Variable Radius Sampling Scheme for 3D Cartesian k-Space Undersampling Pattern Design. In Proceedings of ISMRM 2016, p1818.
8. Lustig M, Pauly JM. SPIRiT: Iterative Self-consistent Parallel Imaging Reconstruction From Arbitrary k-Space. Magn Reson Med 2010;64:457– 471.
Figures