3466
Automatic segmentation of the aortic arterial wall in a pre-clinical rabbit model of atherosclerosis: preliminary experience with a convolutional neural network1Icahn School of Medicine at Mount Sinai, New York, NY, United States
Synopsis
The task of manually evaluating medical images can be onerous, plagued by subjective bias, and subject to human error. In this study we apply a convolutional neural network (CNN) for automated image segmentation of the atherosclerotic vessel wall, a notoriously challenging and time consuming segmentation task. Our CNN shows a classification accuracy of 90% on testing data, and a intersection over union (IoU) weighted by the number of pixels in each class of 86%, indicating excellent segmentation. Our results suggest that, if appropriately optimized this method has the potential deliver faithful and automatic segmentation of the arterial vessel wall.
Introduction
The task of manually evaluating medical images can be onerous, plagued by subjective bias, and subject to human error. Furthermore, most automated techniques have proven effective only in limited and narrowly defined regimes. Recent advances in neural network algorithms show great promise to improve the performance of automated image segmentation methods, and are quickly approaching performance levels equal to that of humans(1,2). In this study we apply a convolutional neural network (CNN) for automated image segmentation of the atherosclerotic vessel wall, a notoriously challenging and time consuming segmentation task(3-5). While here we test this approach to segment the aortic wall in a pre-clinical rabbit model of atherosclerosis, we foresee that it may be applied in the future for segmentation of the vessel wall in other animal models, and in atherosclerotic patients.Methods
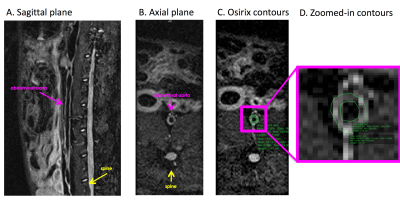
Atherosclerosis was induced in 11 male New Zealand White (NZW) rabbits as previously validated(6). Rabbits were imaged 4 months after HFD initiation on a 3T Siemens mMR clinical scanner (Siemens Healthineers, Erlangen, Germany). A T2 weighted SPACE (Sampling Perfection with Application optimized Contrasts using different flip angle Evolution) sequence (7) was used to acquire black blood images of the arterial vessel wall at an isotropic resolution of 0.6 x 0.6 x 0.6 mm3 (Figure 1). Inner and outer vessel wall contours were traced in Osirix (http://www.osirix-viewer.com) by an expert reader, for each axial slice, from the left renal artery to the iliac bifurcation (Figure 1). A custom made Matlab (https://www.mathworks.com/) program prepared images and contours for CNN training and testing, by creating a mask (label) from Osirix contours (Figure 2). Labeled images were automatically cropped to a predefined size around the vessel wall centroid, for memory and computational limitations during training. Images cropped in the same fashion were used as the ground truth. The final training dataset, encompassing 10 cases and a total of 2707 imaging slices and contours, was trained on a conventional 3 layer convolutional neural network with relu max-pooling layers, and ending with transposed convolution layers conforming to a conventional segmentation model. The network was trained for 300 epochs in batches of 64 using 32 filters with a learning rate of 0.01 and a stride size of one. Finally, noise was removed by deleting all but the largest segmented connected component region in the final segmented image. A standard cross entropy function was used to compute the loss metric. Following training, the model was tested on 1 separate dataset of 260 images to assess performance on unseen data.Results
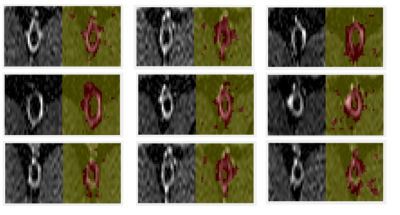
Figure 3 shows representative segmentation results in the training dataset. In each panel, the left side shows the ground truth images, while the right side shows the segmentation results, with the segmented vessel wall in red, and the background in green. In most cases the segmentation algorithm was able to correctly identify the arterial vessel wall, and background regions. Automatic segmentation results of the testing dataset were compared to manual tracings as the ground truth. CNN performance was evaluated by computing: 1) global accuracy: ratio of correctly classified pixels to the total number of pixels, regardless of class; 2) mean accuracy: average ratio of correctly classified pixels to the total number of pixels in each class; 3) mean intersection over union (IoU): ratio of correctly classified pixels to the total number of ground truth and false positives, across classes 4) weighted IoU: average IoU of each class, weighted by the number of pixels in each class; 5) mean boundary F1 (bf) score, indicating how well the predicted boundary of each class aligns with the true boundary, across all classes and all images. For our testing dataset we found a global and mean class accuracy of 90%, indicating excellent capabilities of the network to classify pixels as correctly belonging to the vessel wall. Mean IoU was 63% indicating the presence of a significant number of false positives. However, IoU weighted by the number of pixels in each class, a parameter better suited to evaluate classes of disparate sizes, was 86%, indicating again excellent segmentation.Discussion
We show here a promising approach for automatic segmentation of the arterial vessel wall using a convolution neural network. Our results suggest that, if appropriately optimized this method has the potential deliver faithful and automatic segmentation of the arterial vessel wall. We foresee that such an approach may be helpful in the near future to streamline the labor-intensive image analysis of extensive pre-clinical studies, or for a more unbiased and objective analysis of images in pre-clinical and clinical drug trials.Acknowledgements
No acknowledgement found.References
1. Akkus Z, Galimzianova A, Hoogi A, Rubin DL, Erickson BJ. Deep Learning for Brain MRI Segmentation: State of the Art and Future Directions. J Digit Imaging 2017;30:449-459.
2. Kim YK, Na KS. Application of machine learning classification for structural brain MRI in mood disorders: Critical review from a clinical perspective. Prog Neuropsychopharmacol Biol Psychiatry 2018;80:71-80.
3. Gao S, van 't Klooster R, Kitslaar PH et al. Learning-based automated segmentation of the carotid artery vessel wall in dual-sequence MRI using subdivision surface fitting. Med Phys 2017;44:5244-5259.
4. van 't Klooster R, de Koning PJ, Dehnavi RA et al. Automatic lumen and outer wall segmentation of the carotid artery using deformable three-dimensional models in MR angiography and vessel wall images. J Magn Reson Imaging 2012;35:156-65.
5. van 't Klooster R, Naggara O, Marsico R et al. Automated versus manual in vivo segmentation of carotid plaque MRI. AJNR Am J Neuroradiol 2012;33:1621-7.
6. Calcagno C, Cornily JC, Hyafil F et al. Detection of neovessels in atherosclerotic plaques of rabbits using dynamic contrast enhanced MRI and 18F-FDG PET. Arterioscler Thromb Vasc Biol 2008;28:1311-7.
7. Mihai G, Winner MW, Raman SV, Rajagopalan S, Simonetti OP, Chung YC. Assessment of carotid stenosis using three-dimensional T2-weighted dark blood imaging: Initial experience. J Magn Reson Imaging 2012;35:449-55.
Figures