3465
Quantitative and Noninvasive MRI of the Endothelial Permeability and Function in Carotid Atherosclerosis1Biomedical Engineering, King's College London, London, United Kingdom, 2King's College London, London, United Kingdom, 3Pontificia Universidad Católica de Chile, Santiago, Chile
Synopsis
Over the past two decades, the central role of the endothelium in the initiation, progression, and clinical sequelae of atherosclerosis has been recognized. Increased endothelial permeability and impaired function
Introduction:
Despite the successful implementation of prevention strategies atherosclerosis remains the primary etiologic factor-underlying stroke, that it is one of major causes of premature death and lifelong disability [1]. Compromised structural integrity of the endothelium and higher microvessel density increase vascular permeability that precedes and portents the development of atherosclerosis and its clinical manifestations [2, 3]. The feasibility of quantitative assessment of albumin leakage into the plaque was demonstrated, using a clinically approved albumin-binding contrast agent, in animal models and was associated with lesion progression and instability [4-7]. Here, we sought to translate this technique in man to test whether endothelial permeability is associated with plaque burden and instability in patients with carotid atherosclerosis scheduled for endarterectomy.Material and Methods:
In vivo MRI: Patients with carotid atherosclerosis scheduled for endarterectomy were imaged using a 3.0 T Philips Intera Scanner and a 16-channel head-neck coil. MRA was used to visualize the vasculature and plan the subsequent scans. Zoom T1-black-blood (BB), late gadolinium enhancement (LGE) and T1 maps were acquired before and 20min after bolus injection of MultiHance (0.2 mmol/kg), an albumin-binding contrast agent. Axial, 2D, ECG triggered T1BB images were acquired with: TR= 2 heartbeats; TE=10ms; profile order=low-high; echo train length=6, inversion delay of 350ms, FOV=120×61×43mm, matrix=220×108, resolution=0.5×0.5mm, slice thickness=3mm and flip angle=90o. A 2D Look-Locker sequence was used to determine the inversion time for blood signal nulling. Non-ECG gated 3D LGE axial images were acquired with: TR/TE=10/3.1ms, FOV=120×160×43mm, matrix=224×224, resolution=0.5×0.5mm, slice thickness=4mm, repetition time between subsequent inversion-recovery pulses, 1000ms and flip angle=30°. T1-mapping was performed using a 3D Modified Look-Locker Inversion Recovery (MOLLI) with a segmented k-space acquisition that allows acquisition of T1 maps at a higher spatial resolution crucial for vessel wall mapping. The 3D MOLLI employs two acquisition trains, each preceded by a nonselective inversion pulse with inversion times ranging from 20 to 2000ms, followed by eight segmented readouts for eight individual images. T1 mapping parameters were: TR/TE=5.6/2.8ms, FOV=120×160×43, matrix=120×160, resolution=1×1mm, slice thickness=3mm, flip angle=35°. The T1 maps were automatically generated using a 3-parameter fit model. The images were imported in Osirix and the vessel wall was manually segmented to calculate the IPH and calcification volumes (cm3) and the T1/R1 values. Histology: The endarterectomy sample was collected, marked on the anterior/proximal site and used for histology. Trichrome staining and albumin immunohistochemistry were performed.Results and Discussion:
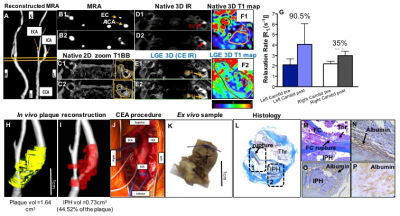
CASE 1: The MR angiogram showed the stenosis of the left internal and external arteries. Native T1BB showed the plaque and native inversion-recovery (IR) images identified the presence of intraplaque haemorrhage (IPH) as hyperintense signal. Native T1 mapping showed the baseline T1 values of the vessel wall while LGE (CE IR) images showed the uptake of agent. Quantitative comparison of vessel wall R1, before and after contrast agent injection, showed a higher increase in the symptomatic carotid artery compared to the contralateral right carotid artery that also had atherosclerotic disease. Reconstructed angiograms fused with the segmented plaque and IPH showed the volume of the lesion and the IPH, respectively. Volumetric analysis showed an extensive IPH that covered ~42% of the symptomatic plaque. The excised plaque and corresponding histology showed a ruptured carotid plaque with superimposed thrombus and intraplaque albumin.
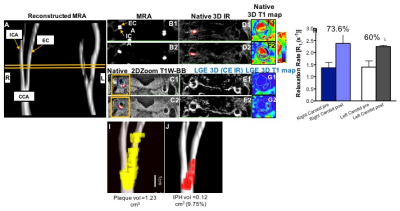
CASE 2: MR angiograms showed the site of stenosis in the right external carotid artery. Native T1BB images showed a more stenotic plaque in the right and a smaller plaque in the left carotid artery. The hyperintense signal on the native IR images signified the presence of IPH in the right carotid artery. Native T1 mapping of the lesion showed the baseline values of the vessel wall. LGE images showed uptake of agent in both the right and left carotid plaques. Quantitative comparison of the vessel wall R1 relaxation rate, before and after contrast agent administration, showed a higher permeability and contrast uptake in the more severely diseased right carotid artery compared to the contralateral vessel. Reconstructed angiograms fused with the segmented plaque and IPH, showed the extent of the lesion and the IPH, respectively.
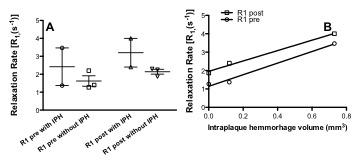
Comparing Cases 1 and 2, the symptomatic lesion associated with a stroke had the highest endothelial permeability (percent increase in R1) and the largest IPH volume (Fig. 3). In both cases, the contralateral vessel showed lower permeability compared to the primary lesion in each patient.
Conclusions:
Non-invasive, quantitative measurement of endothelial permeability, using an albumin-binding contrast agent, may allow assessment of focal atherosclerotic disease burden. Since IPH requires increased plaque permeability, these data suggest that changes in vessel wall R1 due to leakage of the albumin-binding agent could be used as a surrogate marker of plaque progression and future development of IPH.Acknowledgements
National Institute of Health and Research, Cardiovascular Healthcare Technology Co-operative, Pump-Priming Award, United Kingdom.References
1. Writing Group, M., et al., Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation, 2016. 133(4): p. 447-54.
2. Deanfield, J.E., J.P. Halcox, and T.J. Rabelink, Endothelial function and dysfunction: testing and clinical relevance. Circulation, 2007. 115(10): p. 1285-95.
3. Halcox, J.P., et al., Prognostic value of coronary vascular endothelial dysfunction. Circulation, 2002. 106(6): p. 653-8.
4. Lobbes, M.B., et al., Gadofosveset-enhanced magnetic resonance imaging of human carotid atherosclerotic plaques: a proof-of-concept study. Invest Radiol, 2010. 45(5): p. 275-81.
5. Phinikaridou, A., et al., Increased Vascular Permeability Measured With an Albumin-Binding Magnetic Resonance Contrast Agent Is a Surrogate Marker of Rupture-Prone Atherosclerotic Plaque. Circ Cardiovasc Imaging, 2016. 9(12).
6. Phinikaridou, A., et al., Noninvasive MRI monitoring of the effect of interventions on endothelial permeability in murine atherosclerosis using an albumin-binding contrast agent. J Am Heart Assoc, 2013. 2(5): p. e000402.
7. Phinikaridou, A., et al., Noninvasive magnetic resonance imaging evaluation of endothelial permeability in murine using an albumin-binding contrast agent. Circulation, 2012. 126(6): p. 707-19.
Figures