3459
Decreased plaque microvasculature in symptomatic carotid plaques: a DCE-MRI studyGeneviève Crombag1,2, Raf van Hoof1, Floris Schreuder3, Martine Truijman4, Tobien Schreuder5, Narender van Orshoven6, Werner Mess2,7, Paul Hofman1, Robert van Oostenbrugge2,4, Joachim Wildberger1,2, and Eline Kooi1,2
1Radiology & Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 2Cardiovascular Research Institute Maastricht, Maastricht, Netherlands, 3Department of Neurology & Donders Institute for Brain Cognition & Behaviour, Radboud University Medical Centre, Nijmegen, Netherlands, 4Neurology, Maastricht University Medical Center, Maastricht, Netherlands, 5Neurology, Zuyderland Medical Center, Sittard, Netherlands, 6Zuyderland Medical Center, Sittard, Netherlands, 7Clinical Neurophysiology, Maastricht University Medical Center, Maastricht, Netherlands
Synopsis
Rupture of a vulnerable atherosclerotic plaque can lead to thrombus formation and, subsequently, to ischemic events. Intraplaque microvessels are thought to play an important role in atherogenesis, since they may facilitate entrance of red blood cells and inflammatory cells into the plaque tissue due to increased endothelial permeability. Symptomatic patients underwent DCE-MRI to assess plaque microvasculature. A significantly lower vessel wall Ktrans was found in the symptomatic carotid plaque compared to the contralateral asymptomatic side. The decrease in vasa vasorum in the symptomatic plaques might be due to a higher amount of necrotic tissue on this side.
Introduction
Atherosclerosis of the carotid artery accounts for about a fifth of ischemic strokes (1). Rupture of a vulnerable atherosclerotic plaque can lead to thrombus formation and, subsequently, to ischemic events (1, 2). During recent years, identification of patients with an increased stroke risk has turned from plaque stenosis severity to identifying plaque composition (3, 4). Intraplaque microvessels are thought to play an important role in atherogenesis, since they may facilitate entrance of red blood cells and inflammatory cells into the plaque tissue due to increased endothelial permeability (5). Several studies demonstrated the pharmacokinetic parameter Ktrans obtained from dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) can be used to quantify plaque neovasculature noninvasively (6). It was recently demonstrated that patients with cardiovascular events demonstrated a larger adventitial Ktrans, but the same study also showed that there was no significant difference in adventitial Ktrans in the ipsilateral symptomatic plaque versus the contralateral asymptomatic plaque in patients with documented cerebrovascular ischemic events (n=20) (6,7). The objective of our study is to investigate whether there is a difference between Ktrans between the symptomatic (ipsilateral) and asymptomatic (contralateral) carotid plaque in a relatively large study.Materials & Methods
88 symptomatic patients with >2mm ipsilateral carotid plaque underwent 3T MRI (Achieva; Philips Healthcare, Best, the Netherlands; or Discovery MR 750; GE Healthcare, Milwaukee, Wisconsin) using a dedicated 8-channel carotid RF coil (Shanghai Chenguan Medical Technologies Co., Shanghai, China). DCE-MRI was used to assess plaque microvasculature. A contrast medium (CM), Gadobutrol (Gadovist, Bayer Healthcare, Berlin, Germany) using a dose of 0.1 mmol/kg body weight, was injected with a power injector (Spectris Solaris, Medrad, Warrendale, PA, USA) with a rate of 0.5ml/second followed by a 20 ml saline bolus at that same rate. The DCE-MRI acquisition was continued for six minutes after contrast injection.Ktrans the contrast medium transfer rate from the microvasculature to the extracellular space, was calculated for the entire symptomatic and contralateral asymptomatic plaque, using a pharmacokinetic model (Patlak) (8). A multisequence MRI protocol including pre-and post-contrast T1 weighted (T1w) quadruple inversion recovery (QIR) turbo spin echo (TSE) MR imaging was used to identify the presence of a lipid-rich-necrotic-core (LRNC). The inner and outer vessel wall contours of the ipsilateral symptomatic and the contralateral carotid plaque on all slice positions were delineated. The total vessel wall area was divided by the sum of the luminal and vessel wall area, resulting in the normalized wall index (NWI), an indicator of plaque burden. A lipid-rich necrotic core (LRNC) was identified as a region within the bulk of the plaque that did not show contrast enhancement on the post-contrast T1W quadruple inversion recovery (QIR) images. Differences in Ktrans between the symptomatic and asymptomatic side were assessed using a paired samples T-test. Independent samples T-test was used to compare means between plaques with and without LRNC.Results
A significantly lower vessel wall Ktrans was found in the symptomatic carotid plaque compared to the asymptomatic side (0.062±0.0017 min-1 versus 0.057±0.0020 min-1, p=0.033). Mean Ktrans of the adventitia showed no significant difference between the two sides (symptomatic 0.058±0.0020 min-1 versus asymptomatic 0.061±0.0028 min-1, p=0.29). Furthermore, there was a significant lower Ktrans in plaques with a LRNC (0.061±0.017 min-1 versus 0.054±0.014 min-1, p=0.041).Discussion
This study confirms earlier results by Wang et al that there is no difference in advential Ktrans of ipsilateral symptomatic versus contralateral asymptomatic plaque in patients with cerebrovascular ischemic events. In addition, we found a significant lower Ktrans of the entire vessel wall on the symptomatic side compared to the asymptomatic side in symptomatic stroke individuals. Furthermore, there was a significant lower mean Ktrans in plaques with a LRNC. Our finding of lower values of plaque microvasculature in symptomatic plaques are unexpected, since microvasculature has been proposed as a vulnerable plaque characteristic. The decrease in vasa vasorum in the symptomatic plaques might be due to a higher amount of necrotic tissue on this side.Conclusion
The symptomatic carotid plaque shows a significantly lower Ktrans, indicative for a decrease of leaky plaque microvasculature in symptomatic plaques. This may be related to a larger amount of necrotic tissue in symptomatic plaques.Acknowledgements
No acknowledgement found.References
1. Saam T, Hetterich H, Hoffmann V, Yuan C, Dichgans M, Poppert H, et al. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol. 2013;62(12):1081-91.2. Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the Vulnerable Plaque. Journal of the American College of Cardiology. 2006;47(8):C13-C8.
3. Altaf N, Kandiyil N, Hosseini A, Mehta R, MacSweeney S, Auer D. Risk factors associated with cerebrovascular recurrence in symptomatic carotid disease: a comparative study of carotid plaque morphology, microemboli assessment and the European Carotid Surgery Trial risk model. J Am Heart Assoc. 2014;3(3):e000173.
4. Kwee RM, van Oostenbrugge RJ, Hofstra L, Teule GJ, van Engelshoven JM, Mess WH, et al. Identifying vulnerable carotid plaques by noninvasive imaging. Neurology. 2008;70(24 Pt 2):2401-9. 5. Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25(10):2054-61.
6. Wang J, Chen H, Sun J, Hippe DS, Zhang H, Yu S, et al. Dynamic contrast-enhanced MR imaging of carotid vasa vasorum in relation to coronary and cerebrovascular events. Atherosclerosis. 2017.
7. Wang J, Chen H, Sun J, Hippe DS, Zhang H, Yu S, et al. Dynamic contrast-enhanced MR imaging of carotid vasa vasorum in relation to coronary and cerebrovascular events. Atherosclerosis. 2017.
8. van Hoof RH, Heeneman S, Wildberger JE, Kooi ME. Dynamic Contrast-Enhanced MRI to Study Atherosclerotic Plaque Microvasculature. Curr Atheroscler Rep. 2016;18(6):33.
Figures
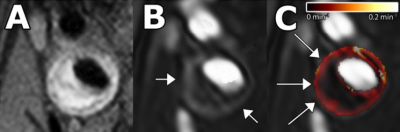
A. Black-blood
T1-weighted turbo-spin-echo image. B. Three-dimensional T1-weighted
fast-field-echo dynamic contrast-enhanced image, acquired six minutes after
contrast injection. The vessel lumen appears bright compared to the
atherosclerotic plaque and surrounding tissues. A ring of enhancement can be
observed at the outer part of the vessel wall (arrows), which is attributed to
the microvasculature. C. Parametric Ktrans map overlaid on DCE-MRI
image. Voxelwise determined Ktrans values are color encoded from 0
to 0.2 min-1. The lipid-rich-necrotic-core exhibits low Ktrans
values (dark). The vascularized adventitia demonstrates high Ktrans
values (red regions, arrows).
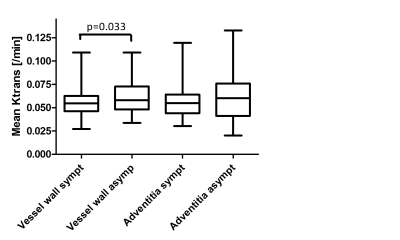
Plot showing a significantly higher mean vessel wall Ktrans in the
asymptomatic (asympt) carotid plaque compared to the symptomatic (sympt) side, while no difference in adventitial Ktrans
is observed.
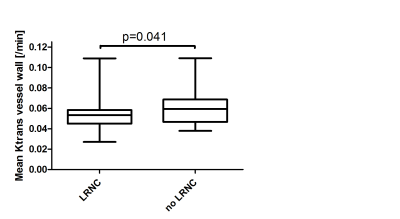
Boxplot shows a
significant difference in Ktrans using an independent samples t-test between
plaques without a LRNC (mean±SEM; 0.061±0.0027min-1) and[EK1] plaques with a LRNC (mean±SEM;0.054±0.0021min-1).