3458
An inverse association between microvasculature and intraplaque hemorrhage in atherosclerotic carotid lesions: an MR imaging study1Radiology & Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 2Cardiovascular Research Institute Maastricht, Maastricht, Netherlands, 3Department of Neurology & Donders Institute for Brain Cognition & Behaviour, Radboud University Medical Centre, Nijmegen, Netherlands, 4Neurology, Maastricht University Medical Center, Maastricht, Netherlands, 5Pathology, Maastricht University Medical Center, MAastricht, Netherlands, 6Neurology, Amsterdam Medical Center, Amsterdam, Netherlands, 7Clinical Neurophysiology, Maastricht University Medical Center, Maastricht, Netherlands, 8Surgery, Maastricht University Medical Center, Maastricht, Netherlands, 9Pathology, Amsterdam Medical Center, Amsterdam, Netherlands
Synopsis
The presence of intraplaque haemorrhage (IPH) has been related to plaque rupture, plaque progression, and predicts cerebrovascular events. However, the mechanisms leading to IPH are not fully understood. The dominant view is that IPH is caused by leakage of erythrocytes from immature microvessels. 101 patients underwent MRI of the symptomatic carotid plaque for detection of IPH and dynamic contrast-enhanced MRI for assessment of plaque microvasculature. A decreased vessel wall Ktrans was found for IPH positive patients. No difference in adventitial Ktrans was found in patients with and without IPH. Not only leaky plaque microvessels, but additional factors may contribute to IPH development.
Introduction
Rupture of a vulnerable
atherosclerotic plaque is likely to be an important cause of clinical ischemic
events such as stroke or myocardial infarction (1). The presence of intraplaque
haemorrhage (IPH) has been related to plaque rupture, is associated with plaque
progression, and predicts cerebrovascular events. However, the mechanisms
leading to IPH are not fully understood. The dominant view is that IPH is
caused by leakage of erythrocytes from immature microvessels. The aim of the
present study was to investigate whether there is an association between
atherosclerotic plaque microvasculature and presence of IPH in a relatively
large prospective cohort study of patients with symptomatic carotid plaque.
Materials & Methods
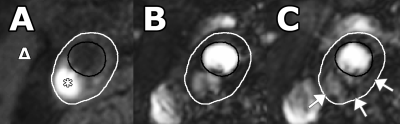
101 symptomatic patients with ≥2mm carotid plaque underwent 3T MRI scanning (Achieva, Philips Healthcare, Best, The Netherlands) using a dedicated 8-channel carotid RF coil (Shanghai Chenguan Medical Technologies Co., Shanghai, China). A T1weighted (T1w) inversion recovery turbo field echo (IR-TFE) sequence was used for detection of IPH. An end diastolic ECG-gated 3D T1-TFE dynamic contrast MR imaging was used for the assessment of plaque microvasculature. The temporal resolution was ~20 seconds/time frame (dependent on heart rate). At the beginning of the third time frame, 0.1 mmol/kg of a small molecular contrast medium Gadobutrol (Gadovist, Bayer HealthCare, Berlin, Germany), was injected with a power injector (Spectris Solaris, Medrad, Warrendale, PA, USA) at 0.5 ml/sec followed by a 20 ml saline flush at the same rate. DCE-MRI acquisition was continued for six minutes after contrast injection. Ktrans, an indicator of microvascular flow, density and leakiness, was estimated using pharmacokinetic modeling (Patlak model) in the vessel wall and adventitia. The entire vessel wall region is defined as the region between the luminal and outer wall contours. The adventitial region of the vessel wall was delineated according to previously described criteria, i.e. all pixels within 0.625 mm of the outer wall contour in a region of the vessel wall with plaque (defined as having a wall thickness >1.5 mm) (2). Statistical analysis was performed using an independent samples T-test and logistic regression, correcting for clinical risk factors.Results
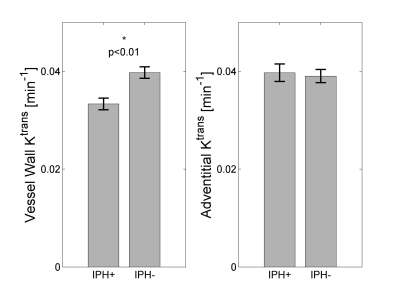
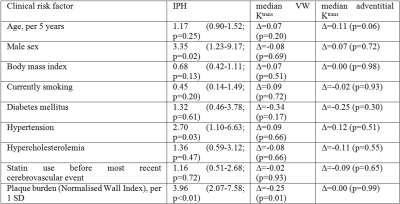
A decreased vessel wall Ktrans was found for IPH positive patients (0.033±0.001 min-1 versus 0.040±0.001, p=0.001; Figure 3), which remained significant after correction for clinical risk factors associated with IPH or Ktrans (Table 1). No difference in adventitial Ktrans was found in patients with and without IPH (0.040±0.002 versus 0.039±0.001 min-1).Discussion
We did not find a positive association between (leaky) microvasculature (Ktrans) and IPH. We found lower median Ktrans values in the entire vessel wall in patients with IPH. This may be related to a larger amount of necrotic tissue in plaques with IPH. Indeed a significant, negative Pearson’s correlation coefficient (ρ=-0.26, p<0.01) was found for median vessel wall Ktrans with the volume % of the lipid-rich necrotic core (LRNC). Our findings are in contrast to the dominant view that IPH is mainly caused by leakage of erythrocytes of plaque microvasculature. Alternatively, disruptions of the fibrous cap (e.g. plaque fissures or plaque rupture) can lead to IPH (4). A histopathological study (5) showed that fibrous cap fissures were frequently accompanied by IPH. In line with the concept that IPH can also originate from fibrous cap disruption, it was recently shown that the presence of intraplaque haemorrhage is associated with a disruption of the atherosclerotic plaque surface (plaque ulceration and/or a fissured fibrous cap) in patients with a mild to moderate carotid stenosis (6).Conclusion
A reduced vessel wall Ktrans is associated with the presence of IPH, independent of clinical risk factors, while no difference in adventitial Ktrans was found in plaques with and without IPH. Thus, we could not confirm a positive association between plaque microvasculature and IPH several weeks after a cerebrovascular event. Not only leaky plaque microvessels, but additional factors may contribute to IPH development.Acknowledgements
Acknowledgements:
The
authors thank R.J. van der Geest (Department of Radiology, Leiden University
Medical Center, Leiden, The Netherlands) for providing the VesselMASS analysis
software package.
Sources of Funding:
This research was performed within the framework of CTMM, the Center for
Translational Molecular Medicine (www.ctmm.nl), project PARISk (grant 01C-202),
and supported by the Dutch Heart Foundation.
This project has
received funding from the European Union (EU) Horizon 2020 research and
innovation programme under the Marie Skłodowska-Curie grant agreement No 722609.”
J.E. Wildberger and M.E. Kooi are supported by Stichting de Weijerhorst.
Disclosures: None.
References
1. Bentzon
JF et al. Circ Res. 2014;114(12):1852-66.
2. Sun J et al. Stroke.
2013;44(4):1031-6.
3. Virmani R et al.
Arterioscler Thromb Vasc Biol. 2005;25(10):2054-61.
4. Falk E. Br Heart J.
1983;50(2):127-34.
5. Constantinides P. Journal
of Atherosclerosis Research. 1966;6(1):1-17.
6. van Dijk AC et al. AJNR Am
J Neuroradiol. 2015;36(11):2127-33.
Figures