3457
High Spatiotemporal Resolution Dynamic Contrast Enhanced (DCE) For Assessing Vascular Inflammation: Initial Clinical Experience1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Department of Bioengineering, University of California, Los Angeles, Los Angeles, CA, United States, 3Department of Medicine, University of California, Los Angeles, Los Angeles, CA, United States, 4Department of Radiology, Anzhen Hospital, Beijing, China
Synopsis
Dynamic contrast enhanced (DCE) MRI is a promising technique for quantitatively assessing the inflammation of atherosclerosis. However, current applications are facing demanding sampling challenges, and compromises have to be made among spatial resolution, coverage and temporal resolution. We recently proposed a 3D DCE protocol based on Low Rank Tensor (LRT) framework to achieve high spatiotemporal resolution, adequate anatomical coverage and dynamic T1 mapping. In this work, we demonstrated the in vivo feasibility on both healthy subjects and patients with known atherosclerosis.
Introduction
Dynamic contrast enhanced (DCE) is a promising method in assessing the density and permeability of the vasa vasorum, which plays a significant role in vascular inflammation and progression of atherosclerosis1. However, applying DCE to evaluate vascular pathology remains technically challenging: 1) it is hard to achieve high spatial and temporal resolution and adequate anatomical coverage simultaneously, so current methods sacrifice one or more of these aspects2,3; 2) the linearity assumption between signal intensity and contrast agent concentration may not allow accurate quantification in areas with high contrast agent uptake4. Recently we developed a novel DCE method based on low-rank tensor (LRT) framework5, achieving 3D high spatiotemporal resolution with entire carotid artery coverage6. In this paper we will report our preliminary in vivo results using this technique.Methods
Sequence design and image reconstruction: As described previously6, continuous scanning with non-selective SR prep pulses followed by FLASH readout was employed to generate T1 contrast information. A 3D Cartesian trajectory with randomized ordering in ky and kz was implemented with the center k-space line acquired every 8 readout lines to form LRT subspace training data5. For reconstruction, the image was formed as a three-way tensor $$$\mathscr{A}$$$ with spatial dimension $$$\mathbf{r}=(x,y,z)$$$, a SR dimension $$$\tau$$$, and a DCE dimension t, and then factorized as $$$\mathbf{A}_{(1)}=\mathbf{U\Phi}$$$. $$$\mathbf{\Phi}$$$, which contains temporal coefficient, is first determined from the subspace training data. $$$\mathbf{U}$$$, which contains spatial coefficients, is then recovered by fitting $$$\mathbf{\Phi}$$$ to the remainder of the sparsely sampled data.
Motion removal: Abrupt patient motion is a major challenge for vessel wall DCE due to the small anatomical structure. In LRT framework, abrupt-motion frames are less correlated to the remainder of the images, corresponding to a higher residual after LRT estimation. The residual $$$\boldsymbol{r}_{res}$$$ is calculated by: $$\boldsymbol{r}_{res}=\sum\nolimits_{i}|(\mathbf{D}_{\rm{tr}}-\mathbf{D}_{\rm{tr}}\mathbf{\Phi}^\dagger\mathbf{\Phi})_{ij}|^2,$$Where $$$\mathbf{D}_{\rm{tr}}\in\mathbb{C}^{N_r\times N_t}$$$ is the matrix form of subspace training data. $$$N_r$$$ and $$$N_t$$$ represent the total number of voxels and time points, respectively. High-residual time points were removed and recovered by LRT framework during the recovery of subspace training data inherently.
Imaging protocol: We focused on achieving dynamic T1 mapping at 0.7mm isotropic spatial resolution, 0.59s temporal resolution and coverage of the entire carotid vasculature. All data were acquired on a 3T commercial MR scanner (MAGNETOM Verio, Siemens Healthineers, Erlangen, Germany). Healthy subjects (n=12) and patients with known carotid atherosclerosis (n = 6) were scanned using the following acquisition parameters: coronal orientation, spatial resolution=0.7mm isotropic, FOV=150x150x26mm3, $$$\alpha =8^\circ$$$, TR/temporal resolution=590ms, scan time=9.8min. Gd contrast media (Gadovist, 0.1mmol/kg) was administered at the rate of 1.0ml/sec followed by 20ml saline flush.
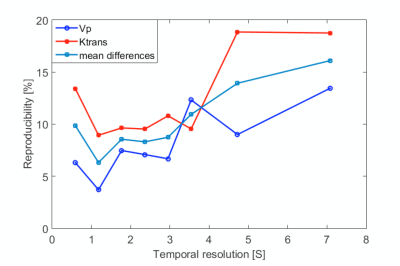
Temporal resolution effect: To evaluate the impact of temporal resolution on the accuracy of kinetic parameter mapping, we evaluate the reproducibility of kinetic parameters at different temporal resolution. In theory, too high temporal resolution may result in noisy concentration curves, while too low temporal resolution may miss the peak of AIF. Two bolus of Gd contrast media was injected using the aforementioned injections scheme, with 30-minutes’ delay time in-between to allow sufficient washout. Temporal resolution reconstructed from 0.59s to 7.2s were evaluated.
Results
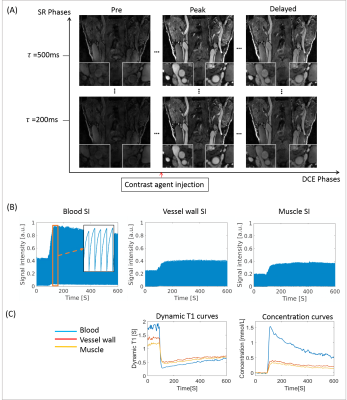
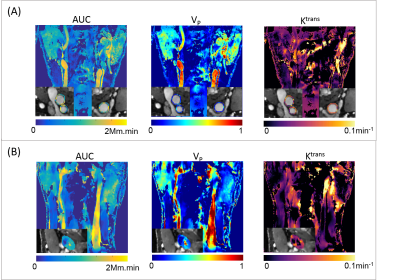
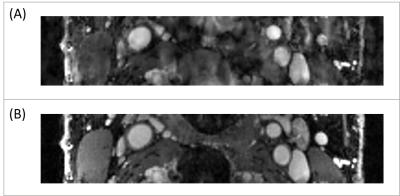
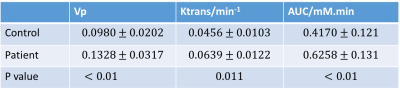
Figure 1(A) shows the typical images of the proposed protocol at different SR/DCE phases, and 1(B) is the conversion from the real-time signal to the contrast agent concentration curve. Figure 2 gives an example of AUC, Vp and Ktrans mapping for a healthy subject and a patient. Figure 3 demonstrates the effect of motion removal. Images after motion removal showed sharper delineation and less artifacts. Figure 4 shows the reproducibility of Vp and Ktrans at different temporal resolution. When temporal resolution = 1.18s, the results between two scans were the most repeatable. Table 1 lists the statistical analysis of AUC, Vp and Ktrans comparingthe healthy subject group and patient group. All the three kinetic parameters were significantly higher in patient group.Discussion
This study showed the in vivo feasibility of the proposed DCE method. Motion removal alleviated the artifacts due to abrupt motion. The evaluation of temporal resolution effect, which sought to balance SNR and accuracy of curve measurement, showed a temporal resolution around 1s had best reproducibility. Patient group had higher AUC, Vp and Ktrans with statistical significance, which was in agreement with the previous published results2,3.Conclusion
In this work, we demonstrated the in vivo feasibility of the proposed DCE method for assessing carotid vessel wall. High spatiotemporal resolution, large anatomical coverage and fully quantitative estimates of contrast media were achieved in healthy subjects and patients with carotid atherosclerosis. Quantification of AUC, Vp and Ktrans was feasible and reproducible. Further clinical validation in a larger patient cohort is warranted.Acknowledgements
No acknowledgement found.References
1 Carlier, S. et al. Vasa vasorum imaging: a new window to the clinical detection of vulnerable atherosclerotic plaques. Current atherosclerosis reports 7, 164-169 (2005).
2 Kerwin, W. S., Oikawa, M., Yuan, C., Jarvik, G. P. & Hatsukami, T. S. MR imaging of adventitial vasa vasorum in carotid atherosclerosis. Magn Reson Med 59, 507-514, doi:10.1002/mrm.21532 (2008).
3 Calcagno, C. et al. Three-dimensional dynamic contrast-enhanced MRI for the accurate, extensive quantification of microvascular permeability in atherosclerotic plaques. NMR Biomed 28, 1304-1314, doi:10.1002/nbm.3369 (2015).
4 Calcagno, C., Mani, V., Ramachandran, S. & Fayad, Z. A. Dynamic contrast enhanced (DCE) magnetic resonance imaging (MRI) of atherosclerotic plaque angiogenesis. Angiogenesis 13, 87-99 (2010).
5 Christodoulou, A.G., Shaw, J.L., Sharif, B., Li, D. A general low-rank tensor framework for high-dimensional cardiac imaging: application to time-resolved T1 mapping. 24th Annual Meeting of ISMRM, Singapore, 2016. Abstract 3032.
6 Wang, N., Christodoulou, AG., Xie, Y., Li, D. Quantitative 3D Dynamic Contrast Enhanced (DCE) Imaging of Carotid Vessel Wall by Fast T1 Mapping. 25th Annual Meeting of ISMRM, Honolulu, HI, USA, 2017. Abstract 7331.
Figures