3447
Rapid, real-time phase-contrast MRI using a combination of radial k-space sampling and compressed sensing with spatially varying regularization weights1Biomedical Engineering, Northwestern University, Evanston, IL, United States, 2Radiology, Northwestern University, Chicago, IL, United States, 3Radiolgy, Northwestern University, Chicago, IL, United States, 4Cardiology, Northwestern University, Chicago, IL, United States
Synopsis
We sought to develop a compressed sensing reconstruction method for radial k-space derived real-time phase contrast that uses spatially varying regularization to reduce flickering artifacts without significant loss in quantified flow accuracy, and evaluate its performance with respect to clinical breath-hold phase contrast in patients undergoing aortic valve evaluation with cardiovascular MRI.
Introduction
Breath-hold phase-contrast (bh-PC) MRI is a proven tool for assessment of blood flow and velocity through the great vessels1. However, as a segmented acquisition, it may produce poor results in patients with irregular heart rhythm and/or dyspnea. One approach to address these problems is to perform real-time PC (rt-PC) MRI, as previously described using radial k-space sampling and compressed sensing (CS)2. This pulse sequence, however, may produce considerable image artifacts (e.g., flicker through time) arising from a high data acceleration rate needed for achieving high temporal resolution (~40 ms). Temporal filters can be used to minimize aliasing artifacts, but such filters may cause temporal blurring and reduce velocity values3,4. In this study, we sought to develop a CS reconstruction method that uses spatially varying regularization to reduce flickering artifacts without significant loss in accuracy, and evaluate its performance against clinical bh-PC MRI in patients undergoing aortic valve evaluation with cardiovascular MRI.Methods
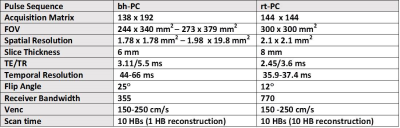
(Patient Enrollment) We scanned 6 patients (4 females, 2 males, mean age = 56.2 ± 17.1 years) with rt-PC and clinical bh-PC MRI on a 1.5T scanner (Siemens, Avanto) to image a plane that is superior to the aortic valve (i.e., ascending aorta). (Pulse Sequence) We developed a rt-PC MRI pulse sequence using radial k-space sampling3 with golden angle ratio = 111.23°5 and compared its performance against clinical bh-PC MRI (see Table 1 for relevant imaging parameters). (Image Reconstruction) The CS reconstruction was performed off-line on a workstation equipped with MATLAB (R2014a, The MathWorks). For CS reconstruction, after nonuniform fast Fourier transform6 and self-calibration7 of coil sensitivity maps, temporal total variation was used as the sparsifying transform8 with normalized regularization weight (λ)= 0.045 (or 4.5% of maximal signal). Note, the reference and velocity-encoded data were reconstructed separately. As shown in Figure 1, we explored two different regularization approaches: static and spatially varying. We adopted a previous described method9 to automatically derive a spatial mask that assigns high λ to locations generating high levels of flickering artifacts arising from the outer periphery (e.g., chest wall) and moderate levels of flickering artifact arising from static body tissues such as the liver (Figure 1). Briefly, locations causing flickering artifacts were detected by first applying a 1D median temporal filter to standard CS reconstructed magnitude images. The difference between the median filtered and original images was averaged over time to derive a normalized threshold value of 0.05 (Figure 1B). To remove flickering artifact arising from static body tissues, such locations were detected by averaging the phase images over time to derive a threshold value of 0.3% of velocity encoding (venc)(Figure 1B). We then combined the magnitude and phase masks with empirically derived factors 4 and 2, respectively, to derive a spatial varying λ map. Note, these empirical factors were derived from training data sets. (Qualitative Assessment) Two cardiologists and one radiologist graded the artifact level for CS reconstruction with static and spatially varying λ on a 5-point Likert scale (1: worst; 3: clinically acceptable; 5: best). (Velocity/Flow Quantification) For each data set, for both bh-PC and rt-PC MRI, a single region of interest (ROI) was drawn manually around the ascending aorta at a frame exhibiting high velocities (i.e., contrast). The ROI was then deformed through time using the Advanced Normalization Tools (ANTS)10. The rt-PC flow curves from multiple heat beats were average and compared to bh-PC flow curves using two tailed paired t-test and linear regression and Bland Altman analyses.Results
The mean artifact score for spatially varying λ was better (3.3 ± 0.8) than that for static λ (2.6 ± 0.5)(see Figure 2 for an example), where only the spatially varying λ was deemed clinical acceptable (score 3). Compared with clinical bh-PC, both static λ and spatially varying λ CS reconstructions produced flow values that were not significantly different (p>0.05). Figure 3 shows the scatter plots resulting from the Bland-Altman and linear regression analyses, which indicate that the flow values are strongly correlated and in good agreement between bh-PC and both CS reconstructions.Conclusion
This study demonstrated a CS reconstruction method that uses spatially varying λ to reduce flickering artifacts arising from highly accelerated rt-PC MRI, without significant loss in accuracy. This reconstruction method produced relatively accurate flow metrics as compared with clinical bh-PC MRI. Future studies in a larger patient cohort are warranted to further evaluate the clinical utility.Acknowledgements
NIH grant: R01HL116895, R01HL138578, R21EB024315, R21AG055954References
1. Kramer CM, Barkhausen J, Flamm SD, et al. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: board of trustees task force on standardized protocols. JCMR. 2008; 10(1):35.
2. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. MRM. 2007 ;58(6):1182-95.
3. Joseph A, Kowallick JT, Merboldt KD, et al. Real‐time flow MRI of the aorta at a resolution of 40 msec. JMRI. 2014; 40(1):206-13.
4. Frahm J, Schätz S, Untenberger M, et al. On the temporal fidelity of nonlinear inverse reconstructions for real-time MRI–The motion challenge. The Open Medical Imaging Journal. 2014; 8:1-7. 5. Winkelmann S, Schaeffter T, Koehler T, et al. An optimal radial profile order based on the Golden Ratio for time-resolved MRI. IEEE transactions on medical imaging. 2007; 26(1):68-76.
6. Fessler JA. On NUFFT-based gridding for non-Cartesian MRI. Journal of Magnetic Resonance. 2007;188(2):191-5
7. Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. MRM. 2000;43(5):682-690.
8. Feng L, Grimm R, Block KT, et al. Golden‐angle radial sparse parallel MRI: Combination of compressed sensing, parallel imaging, and golden‐angle radial sampling for fast and flexible dynamic volumetric MRI. MRM. 2014 ;72(3):707-17.
9. Ilicak E, Cetin S, Bulut E, et al. Targeted vessel reconstruction in non‐contrast‐enhanced steady‐state free precession angiography. NMR in Biomedicine. 2016; 29(5):532-44.
10. Avants BB, Epstein CL, Grossman M, et al. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Medical image analysis. 2008; 12(1):26-41
Figures