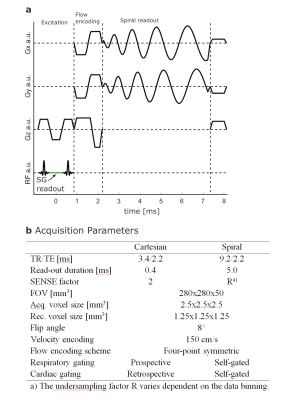
3443
Self-Gated Golden-Angle Spiral 4D Flow MRI1Radiology, University Hospital of Cologne, Cologne, Germany, 2Philips GmbH Healthcare, Hamburg, Germany
Synopsis
A time efficient fully self-gated 4D flow sequence is presented that operates at predictable scan times and allows for a retrospective binning into an arbitrary number of cardiac and/or respiratory states. The acquisition time is fixed independently of the subjects’ physiology. Data is reconstructed using conjugate-gradient-SENSE. Feasibility is shown in 10 healthy volunteers and results are compared to a standard Cartesian 4D flow sequence.
Introduction
4D flow MRI enables a comprehensive analysis of hemodynamic processes in several cardiovascular applications1. However, reaching clinical feasibility remains challenging: The need for simultaneous cardiac and respiratory gating may lead to unpredictable scan times. Furthermore, using external devices such as a vectorcardiogram (VCG) and a respiratory belt require additional patient preparation and contain the risk of failure, e.g. at higher field strengths and in specific patient groups2,3. Using an interleaved MR navigator, such as a pencil beam navigator to track the motion of the liver/diaphragm interface4-6 potentially leads to the disturbance of the signals steady state and prevents the data coverage of the entire cardiac cycle. Self-gating (SG) may overcome these limitations7,8. The aim of the current work was to develop a robust, and time-efficient, 4D flow MRI acquisition sequence operating at a fixed scan time independent of subject specific physiology without the need of external sensors or interleaved MR Navigators.Methods
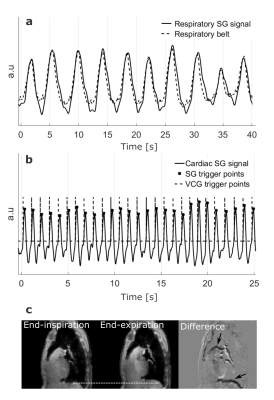
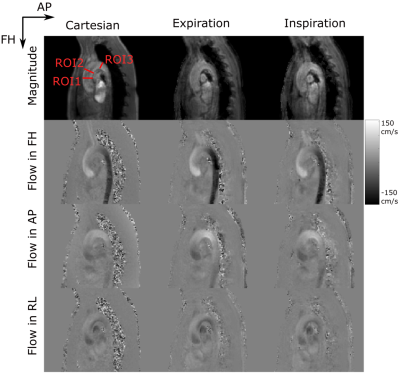
The aortas of 10 healthy volunteers were imaged on a 3T system (Ingenia, Philips, Best, The Netherlands) using a 28 channel array coil. A self-gated golden-angle9 (SGGA) stack-of-spiral acquisition was used. Scan time was set to a fixed value of 15:06 min. Volunteers were equipped with a VCG and a respiratory belt, which were not used for gating in the SGGA sequence. The FID generated at every TR between the spatial-spectral excitation pulses was used as the SG signal10,11 (Figure 1a). The resulting SG signal was eddy-current corrected and band-pass filtered between 0.1 Hz and 0.5 Hz to extract respiratory motion and 0.6 Hz and 3 Hz to extract cardiac motion information12. 949 spiral spokes per volume and flow encoding direction were acquired, incremented by the golden-angle of 222.49° 9. Data were retrospectively re-binned into two breathing states (expiration and inspiration) at a mean temporal resolution of 45.9 ± 4.0 ms. To avoid image artefacts by local k-space undersampling a regularized conjugate gradient SENSE (CG-SENSE) reconstruction was used13,14. For comparison, a conventionally gated Cartesian 4D flow sequence (mean scan time: 13:00 ± 01:46 min, temporal resolution: 45.6 ± 4.0) was acquired using a VCG and pencil-beam navigator for cardiac and respiratory gating, respectively4-6 (Figure 1b). Flow quantification was performed in three ROIs (Figure 3, top left).Results
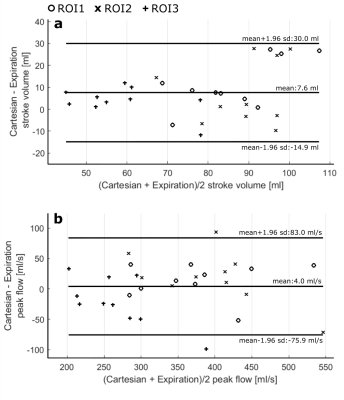
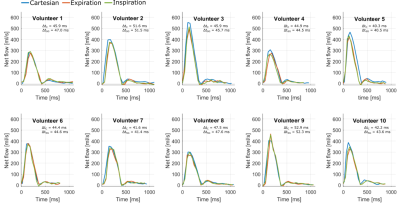
The temporal standard deviation between the VCG and the SG trigger points was 18.6 ± 6.2 ms. Motion information extracted from the SG signals compared to data from the external sensors are shown in figure 2a and b. Figure 2c shows an exemplary reconstruction of one SGGA data set at end-expiration and end-inspiration. Exemplary 4D Flow reconstructions are shown in figure 3. Compared stroke volumes and peak flows over all volunteers and ROIs are summarized in a Bland-Altman plot (Figure 4). Net flow curves generated in ROI1 are shown in figure 5.Discussion
We present a respiratory and cardiac gated 4D flow MRI sequence, utilizing intrinsic SG signals in combination with a spiral read-out and CG-SENSE reconstruction. This sequence allows acquiring 4D flow data at a predictable scan time, independent of respiratory and cardiac motion variations. Deviation between VCG and SG trigger points indicate a high precision in line with previously proposed SG approaches10,12,15,16. Comparison of flow quantities between the two acquisition methods showed good agreement, SV and PF deviations being in-line with previous studies17,18. Observed differences between the 4D flow sequences may be associated to subject specific physiological flow changes and inherent differences of both acquisition and motion gating methods.Conclusion
Overall, the feasibility to acquire respiratory and cardiac gated 4D flow MRI at a predictable scan time using the propose SGGA sequence was demonstrated. It enables the reconstruction of 4D flow data in different breathing states from a single dataset. The analysis of respiratory dependent flow19,20 using this technique is warranted.Acknowledgements
No acknowledgement found.References
1. Markl M, Schnell S, Wu C, Bollache E, Jarvis K, Barker AJ, Robinson JD, Rigsby CK. Advanced flow MRI: emerging techniques and applications. Clin Radiol 2016;71(8):779-795.
2. Nacif MS, Zavodni A, Kawel N, Choi EY, Lima JA, Bluemke DA. Cardiac magnetic resonance imaging and its electrocardiographs (ECG): tips and tricks. Int J Cardiovasc Imaging 2012;28(6):1465-1475.
3. Santelli C, Nezafat R, Goddu B, Manning WJ, Smink J, Kozerke S, Peters DC. Respiratory bellows revisited for motion compensation: preliminary experience for cardiovascular MR. Magn Reson Med 2011;65(4):1097-1102.
4. Ehman RL, Felmlee JP. Adaptive technique for high-definition MR imaging of moving structures. Radiology 1989;173(1):255-263.
5. Markl M, Harloff A, Bley TA, Zaitsev M, Jung B, Weigang E, Langer M, Hennig J, Frydrychowicz A. Time-resolved 3D MR velocity mapping at 3T: improved navigator-gated assessment of vascular anatomy and blood flow. J Magn Reson Imaging 2007;25(4):824-831.
6. van Ooij P, Semaan E, Schnell S, Giri S, Stankovic Z, Carr J, Barker AJ, Markl M. Improved respiratory navigator gating for thoracic 4D flow MRI. Magn Reson Imaging 2015;33(8):992-999.
7. Larson AC, White RD, Laub G, McVeigh ER, Li D, Simonetti OP. Self-gated cardiac cine MRI. Magn Reson Med 2004;51(1):93-102.
8. Larson AC, Kellman P, Arai A, Hirsch GA, McVeigh E, Li D, Simonetti OP. Preliminary investigation of respiratory self-gating for free-breathing segmented cine MRI. Magn Reson Med 2005;53(1):159-168.
9. Winkelmann S, Schaeffter T, Koehler T, Eggers H, Doessel O. An optimal radial profile order based on the Golden Ratio for time-resolved MRI. IEEE Trans Med Imaging 2007;26(1):68-76.
10. Ingle RR, Santos JM, Overall WR, McConnell MV, Hu BS, Nishimura DG. Self-gated fat-suppressed cardiac cine MRI. Magn Reson Med 2015;73(5):1764-1774.
11. Bastkowski R, Weiss K, Maintz D, Giese D. Respiratory self-gated golden-angle spiral 4D flow MRI. In: Proceedings of the 25th Annual Meeting of ISMRM; Honolulu, Hawaii, USA. 2017 April. (3234). 12. Buehrer M, Curcic J, Boesiger P, Kozerke S. Prospective self-gating for simultaneous compensation of cardiac and respiratory motion. Magn Reson Med 2008;60(3):683-690.
13. Pruessmann KP, Weiger M, Bornert P, Boesiger P. Advances in sensitivity encoding with arbitrary k-space trajectories. Magn Reson Med 2001;46(4):638-651.
14. Fessler JA, Sutton BP. Nonuniform fast Fourier transforms using min-max interpolation. Ieee T Signal Proces 2003;51(2):560-574.
15. Thompson RB, McVeigh ER. Flow-gated phase-contrast MRI using radial acquisitions. Magn Reson Med 2004;52(3):598-604.
16. Nijm GM, Sahakian AV, Swiryn S, Carr JC, Sheehan JJ, Larson AC. Comparison of self-gated cine MRI retrospective cardiac synchronization algorithms. J Magn Reson Imaging 2008;28(3):767-772. 17. Uribe S, Beerbaum P, Sorensen TS, Rasmusson A, Razavi R, Schaeffter T. Four-dimensional (4D) flow of the whole heart and great vessels using real-time respiratory self-gating. Magn Reson Med 2009;62(4):984-992.
18. Markl M, Wallis W, Harloff A. Reproducibility of flow and wall shear stress analysis using flow-sensitive four-dimensional MRI. J Magn Reson Imaging 2011;33(4):988-994.
19. Schrauben EM, Anderson AG, Johnson KM, Wieben O. Respiratory-induced venous blood flow effects using flexible retrospective double-gating. J Magn Reson Imaging 2015;42(1):211-216.
20. Korperich H, Barth P, Gieseke J, Muller K, Burchert W, Esdorn H, Kececioglu D, Beerbaum P, Laser KT. Impact of respiration on stroke volumes in paediatric controls and in patients after Fontan procedure assessed by MR real-time phase-velocity mapping. Eur Heart J Cardiovasc Imaging 2015;16(2):198-209.
Figures