3441
Modeling Physiological Flow Variation in Total Cavopulmonary Connection with Physical Model Experiments and 4D Flow MRI1Mechanical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 2Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Medicine, University of Wisconsin-Madison, Madison, WI, United States, 4Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
The total cavopulmonary connection (TCPC) is a successful treatment for single ventricle defect, however, long term complications, such as exercise intolerance still occur. To examine the effects of exercise conditions on TCPC fluid dynamics, in vitro experiments using 4D Flow MRI were conducted at high and low flow conditions. Significant difference in pulmonary flow distribution between conditions was found, and flow patterns and structures were characterized. After further development these models may provide a useful tool for analyzing and predicting changes in a variety of patient specific TCPC anatomy.
Introduction
Congenital heart diseases involving single ventricle physiology are often treated by creating a total cavopulmonary connection (TCPC). [1] This connection successfully facilitates passive return of blood to the pulmonary circulation; however, the hemodynamic alterations can lead to long term complications. [2],[3] Hemodynamics through the patient connection can be studied in vivo with advanced flow imaging techniques. Yet, imaging methods alone cannot predict flow changes that occur due to varying geometric and physiological conditions, such as those seen during exercise. Computational methods can be used simulate these changes, but such methods rely heavily on accurate assumptions and physiologically accurate boundary conditions. [4] In vitro experiments using physical patient specific models may offer an alternative that allows for analysis of real fluid flow. In this study, we examined the hemodynamic changes that occur during high and low flow conditions in physical models of six TCPC patient geometries.Methods
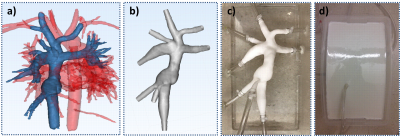
In this IRB-approved and HIPPA-compliant study, 4D Flow MRI was performed on six TCPC subjects with a 3T system (Discovery MR750, GE Healthcare) using contrast (based on body weight) and 3D radially undersampled phase contrast (PC) acquisition (5-point PC-VIPR)[5] with increased velocity sensitivity performance over a large chest imaging volume. The TCPC anatomy of each patient was segmented from PC angiograms using MIMICS (Materialise, Leuven, Belgium) to create a three-dimensional (3D) geometry (Figure 1a). Flow models were designed in 3-matic (Materialise, Leuven, Belgium) (Figure 1b), fabricated (Figure 1c) using a powder bed fusion technique (DTM Sinterstation 2500CI ATC, 3D Systems, INc., Rock Hill, SC, USA), and surrounded by polyurethane resin (Figure 1d) to maximize image quality. Each model was connected to a perfusion pump (Stockert SIII Heart-Lung Machine) using surgical tubing. The model was then placed in the scanner and water was pumped through the model at system flow rates intended to simulate low (2 LPM) and high (3LPM) flow, and then scanned with the PCVIPR. Flow data was then processed in Ensight (CEI, Apex, NC) to obtain flow, velocity, and kinetic energy at each inlet and outlet plane and vorticity, helicity, and flow and particle trace distributions throughout each model. Metrics were compared between low and high flow conditions using a students paired t-test (p<0.05).Results
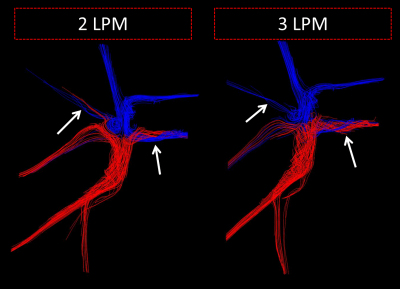
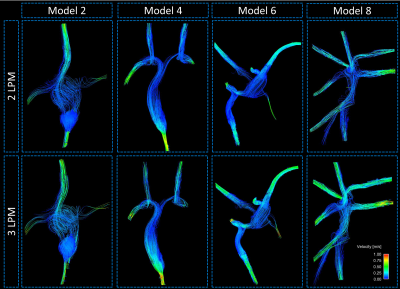
A significant difference in pathline distribution from each vena cava inlet to the pulmonary artery outlets was observed between low and high flow conditions (p=0.03) (Figure 2). Furthermore, total flow distributions between the left and right pulmonary artery outlets varied, though the difference was not significant (p=0.13). The flow patterns and structures are represented by streamlines in Figure 3. Additional metrics of flow patterns, such as vorticity and helicity did not increase with the same magnitude as the flow increase. Lastly, it was observed that strong inflow jets developed at the inlet connections.Discussion
In this study, flow patterns and hemodynamic metrics during low and high flow conditions were analyzed using physical models of anatomically accurate patient vasculature. Results showed that higher flow rates, as seen during exercise, can change flow patterns and fluid distributions in total cavopulmonary connection models. Furthermore, the relationship between increased flow and development of vortical and helical structures was not proportional, suggesting an intricate relationship that warrants further study. If the same hemodynamic alterations occur in vivo, the efficiency of the connection and flow distribution through the pulmonary circulation may be significantly altered in the TCPC patient during exercise. However, several limitations affect the applicability of the results of this study to patient application at this stage of development. First of all, the anatomical models used for these experiments were isolated from upstream and downstream effects that are present in vivo. Future experiments will include adjustable resistance and compliance elements that can be matched with physiological conditions. Additionally, rigid models were used in this study. Although, flow induced motion effects are often small in the area of the TCPC, physiologically realistic wall properties warrant further investigation. The rigid tube perfusion connections also imposed a limitation by creating unrealistically strong flow jets at the vena cava inlets. Future work will incorporate computational simulation with the goal of creating a virtual surgery planning tool that can be validated with these physical model experiments and in vivo patient imaging.Conclusion
Patient specific flow model experiments were conducted to study the effects of simulated exercise conditions on the flow patterns in total cavopulmonary connections. These models will be further developed to create an analysis and planning tool for TCPC.Acknowledgements
The research presented was supported under NIH awards UL1TR000427 and TL1TR000429. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.References
[1] Collins, Ronnie Thomas, Pratik Doshi, Jennifer Onukwube, Ricki Y. Fram, and James M. Robbins. "Risk Factors for Increased Hospital Resource Utilization and In-Hospital Mortality in Adults with Single Ventricle Congenital Heart Disease." The American Journal of Cardiology 118.3 (2016): 453-62.
[2] Khairy, P., S. M. Fernandes, J. E. Mayer, J. K. Triedman, E. P. Walsh, J. E. Lock, and M. J. Landzberg. "Long-Term Survival, Modes of Death, and Predictors of Mortality in Patients With Fontan Surgery." Circulation 117.1 (2008): 85-92.
[3] Lardo, A.C., Webber, S.A., Friehs, I., del Nido, P.J., Cape, E.G., 1999. Fluid dynamic comparison of intra-atrial and extracardiac total cavo-pulmonary connections. J. Thorac. Cardiovasc. Surg. 117. 697-704.
[4] Sundareswaran, K. S., et al. “The total cavopulmonary connection resistance: a significant impact on single ventricle hemodynamics at rest and exercise.” AJP: Heart and Circulatory Physiology, vol. 295, no. 6, 2008, doi:10.1152/ajpheart.00628.2008
[5] Johnson, K. M., Lum, D. P., Turski, P. A., Block, W. F., Mistretta, C. A., and Wieben, O., 2008, "Improved 3D phase contrast MRI with off-resonance corrected dual echo VIPR," Magn Reson Med, 60(6), pp. 1329-1336.
Figures