3439
Magnetic Particle Imaging based 4D flow analysis technique using regional MRI data evaluation – initial in vivo results of a beating rodent heartJochen Franke1,2, Heinrich Lehr1, and Volkmar Schulz2
1Preclinical Imaging, Bruker BioSpin MRI GmbH, Ettlingen, Germany, 2Physics of Molecular Imaging Systems, University RWTH Aachen, Aachen, Germany
Synopsis
A dual-modal cardiovascular in vivo assessment in rodents was performed using a highly integrated Magnetic Particle Imaging – Magnetic Resonance Imaging hybrid system. 4D velocity flow field estimation of a beating rodent heart was extracted from the pulsed tracer information within the MPI dataset of a non-toxic tracer bolus. By means of co-registered morphological MRI data acquired at 0.5 T, an anatomical regional velocity flow field evaluation was performed for the four heart chambers individually.
Purpose:
Quantitative velocity estimation is a common tool in cardiac assessments to screen for or stage cardiovascular diseases. The invention of Magnetic Particle Imaging (MPI), a tracer-based imaging modality presented by Gleich and Weizenecker [1] in 2005, opens up new techniques such as fast, non-invasive 4D velocity field estimation [7]. While multimodal imaging is emerging in modern medicine, raising the diagnostic value of the single modalities by combining their information, MPI and MRI hybrid imaging has been proven to be a promising approach by using two separate stand-alone imaging systems [2], an insert approach [3] as well as in a highly integrated hybrid system topology [4-5]. With such imaging systems morphological information can be used to visualize, interpret as well as analyze the functional information at anatomical landmarks.Methods:
A multi-modal in vivo experiment was performed on the MPI-MRI hybrid system characterized in [5]. By means of the MRI-functionality the subject was precisely positioned to the static 3D MPI field-of-view (FOV) and three orthogonal high spatial resolution morphological slices were acquired (MSME, TE/TR=8.6/300 ms, matrix=128×128×9, FOV=50×50×27, BW=25 kHz, average=25, scan time=16 min). The MPI-functionality (TR=21 ms, ADFx,y,z=12 mT, GSFmax=2.2 T/m, BW=625 kHz, repetitions=30000) was used to observe a 20 µl non-toxic bolus (Resovist, Bayer Schering Pharma AG, Germany) manually administered into the tail vein of an anesthetized rat (Lewis, 262 g body weight). These MPI raw data were reconstructed using a system matrix based image reconstruction algorithm (Kaczmarz iterative algorithm [6], background corrected raw data, AVG=1, 60 iterations, λ=8∙10-3, matrix=28×28×16, FOV=28×28×16 mm³) implemented in the ParaVision6 software framework (Bruker BioSpin, Germany). Deploying an Optical Flow Analysis algorithm [7], 140 reconstructed consecutive MPI data were used to estimate a 4D velocity vector field representing one cardiac cycle. Regional 4D velocity vector field analysis was performed by exploiting anatomical volumes-of-interest, manually segmented from one static co-registered MRI dataset.Results:
Fig. 1 shows one selected time point of the MPI-based 4D velocity vector estimation conjoined with three orthogonal high spatial MRI planes. Time-velocity-curves extracted from the 4D velocity vector field are presented as |v|(t) in Fig. 2 for the four MR-derived volumes-of-interest right atrium (RA), right ventricle (RV), left atrium (LA) and left ventricle (LV).Discussion & Conclusion:
This study demonstrates the system’s capability for sequential dual-modal in vivo imaging. Furthermore, MPI-based 4D velocity vector estimation conjoined with morphological reference MRI datasets with a high confidence of spatial co-registration allows for anatomical regional evaluation. 140 reconstructed consecutive MPI 3D volumes (corresponding to a total data acquisition time of approx. 3 seconds) suffice to estimate a 4D velocity vector field representing one cardiac cycle of a beating rodent heart.Acknowledgements
The authors thankfully acknowledge the financial support by the German Federal Ministry of Education and Research, FKZ 13N11088. All animal experiments were performed with the legal approval of the responsible Animal Ethics Committee (Regional Council of Karlsruhe, Germany; 35-9185.81/G-178/12).References
[1] B. Gleich and J. Weizenecker, Nature, 10.1038/nature03808. [2] J. Weizenecker et al., Physics in Medicine and Biology, 10.1088/0031-9155/54/5/L01 [3] P. Vogel et al., IEEE TMI, 10.1109/TMI.2014.2327515. [4] J. Franke et al., Proc. IWMPI, 10.1109/IWMPI.2013.6528363 [5] J. Franke et al., IEEE TMI (2016): 1993-2004 [6] S. Kaczmarz,. Bull. Internat. Acad. Polon. Sci. Lett., 35:355357, 1937 [7] J. Franke et al., International Journal on Magnetic Particle Imaging, 3(1).Figures
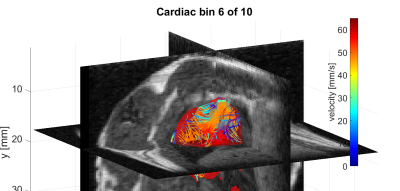
One time point of the MPI-based
4D velocity vector estimation of a beating rodent heart in a color-coded streamline
representation. For morphological guidance, the velocity vector field is conjoined
with three orthogonal high spatial MRI planes (grayscale).
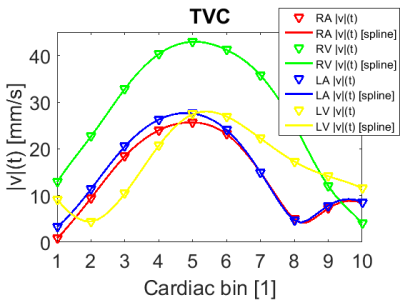
Time-velocity-curves extracted
from the 4D velocity vector field are presented as |v|(t) for the four MR-derived
volumes-of-interest right atrium (RA), right ventricle (RV), left atrium (LA)
and left ventricle (LV) individually.