Lihua Chen1, Ailian Liu1, Yan Guo2, Xin Li3, and Dan Guo1
1The First Affiliated Hospital of DaLian Medical University, Dalian, China, 2GE Healthcare, China, ShenYang, China, 3GE Healthcare, China, GuangZhou, China
Synopsis
Prostate
cancer is the second most common cancer for men, and it has high leading cause
of cancer death among men. The term radiomics has attracted increased attention
in recent years, and it is the process of the conversion of medical images into
high-dimensional, mineable data via high-throughput extraction of quantitative
features, followed by subsequent data analysis for decision support.The
aim of this study was to evaluate radiomics as a tool to distinguish PCa from
BPH based on diffusion-weighted imaging (DWI) sequence without subjective
factors.
Introduction
Prostate
cancer is the second most common cancer for men, and it has high leading cause
of cancer death among men. The term radiomics has attracted increased attention
in recent years, and it is the process of the conversion of medical images into
high-dimensional, mineable data via high-throughput extraction of quantitative
features, followed by subsequent data analysis for decision support [1-2].The
aim of this study was to evaluate radiomics as a tool to distinguish PCa from
BPH based on diffusion-weighted imaging (DWI) sequence without subjective
factors.Purpose
To
evaluate the usefulness of radiomics features indistinguishing prostate cancer
(PCa) from benign prostatic hyperplasia (BPH) based on
diffusion-weighted imaging (DWI) sequence without subjective factors.Materials and Methods
This retrospective study was approved by local
IRB, and written informed consent was waived. 200 patients with
PCa or BPH who underwent MRI exams between January 2010 and February 2017 were
enrolled in this study. Among them, 100 were PCa and 100 were BPH, confirmed by
pathologically. Inclusion criteria were as follows: patients first underwent
MRI exams followed by surgery or biopsy within one month, prior to treatment.
All MRI scans were performed on a 3.0T scanner (GE-Signa
HDXT, Milwaukee) with an eight-channel phased-array
body-coil. DWI maps were extracted using an EPI sequence with b =
1000sec/mm2.High-throughput
extraction and analysis of the radiomic features based on DWI mainly included
five key procedures: 1) data pre-processing and segmentation was performed by
two radiologists, who respectively had 3 years and 1 year experience in
prostate imagingwho were blind for pathology, 2D region of interest (ROI) was
sketched along the edge of the whole prostate gland at the slice with the
maximum diameter of the lesion.2) 397 radiomics features, including size and
shape based–features, histogram and GLCM (Gray-Level Co-occurrence Matrix) as
well as GLRLM (Gray-Level Run Length Matrix) texture features were generated
automatically using Analysis-Kinetics software (GE Healthcare, China). 3)
Statistical analysis was conducted with R software (version
3.3.2;http://www.Rproject.org). Dimensionality reduction was based on t test,
Kruskal-Wallis test and auto-correlation analysis. 4) Model construction: 70
PCa and 70 BPH who were time-dependent selected in 200 patients were used to
supervised Model-learning and Logistic regression model was used. Incorporated
the patients age, DWI signal characteristics and total prostate specific
antigen (TPSA) value, and this was presented with a radiomics nomogram.5) Model
validation: 30 PCa and 30 BPH were used and compared with pathologic diagnosis,
in which confusionmatrix and receiver operating characteristics (ROC) were used
to assess the efficiency of model. Calibration Curve and Decision Curve
were used to evaluate the clinical application value of nomogram.Results
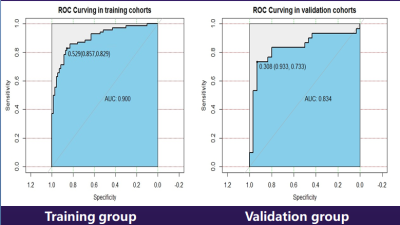
The area under the curve (AUC) of
Logisticregression model in discriminating the two groups for training group
and verification group 0.900 and 0.834, sensitivity were 82.9% and 73.3%,
specificity were 85.7% and 93.3%(fig 1), with 75% diagnosis accuracy rate. The
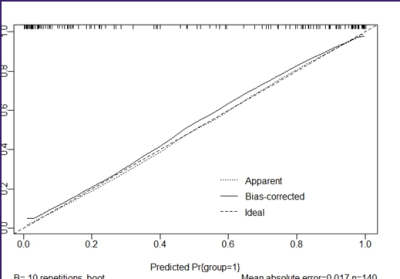
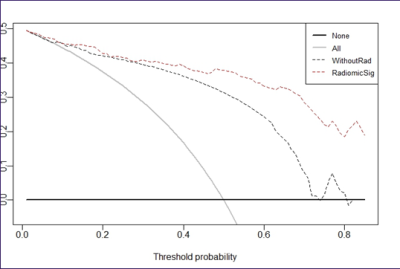
analysis results of Calibration Curve(fig 2) and Decision Curve(fig 3) showed
that nomogram included the clinical parameters has good clinical application
value.Discussion and Conclusion
Compared with traditional manual method, Radiomics
features not only could lighten the visual fatigue for radiologist but also raise
the precision of diagnosis. Radiomics features of DWI performed well indistinguishing
PCa from BPH, which could help objectively and quantitatively evaluate tumor
heterogeneity, and have prospect of being an independent &non-invasive
efficient diagnostic tool.Acknowledgements
No acknowledgement found.References
[1] Aerts HJ, Velazquez ER,
Leijenaar RT, et al: Decoding tumour phenotype by noninvasive imaging using a
quantitative radiomics approach. Nat Commun 5:4006, 2014 [Erratum: Nat Commun
5:4644, 2014].[2]. Gillies RJ, Kinahan PE, Hricak H: Radiomics: Images are more
than pictures, they are data. Radiology 278:563-577, 2016.