3406
Accelerated spin-echo fMRI using generalized SLIce Dithered Enhanced Resolution Simultaneous MultiSlice (gSlider-SMS) with 'complex-basis' RF-encoding1Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 2Department of Biomedical Engineering, Zhejiang Uninversity, Hangzhou, Zhejiang, China, 3Department of Radiology, Harvard Medical School, Boston, MA, United States, 4Medical Engineering & Medical Physics, Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA, United States, 5Vanderbilt University Institute of Imaging Science, Vanderbilt University, Nashville, TN, United States, 6Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA, United States
Synopsis
High spatiotemporal resolution spin-echo (SE) fMRI acquisition is challenging due to the longer repetition times (TR) compared to conventional gradient-echo (GE) fMRI. In this study, we developed a new method, dubbed ‘complex-basis’ gSlider, which utilizes the spatiotemporal phase-smoothness of SE-fMRI time frames to accelerate the slice coverage of SE-fMRI acquisitions. We further combined ‘complex-basis’ gSlider with conventional SMS to boost the slice-acceleration as well. The proposed method showed comparable tSNR and a two-fold increase in slice-acceleration when compared with standard SE-SMS-EPI. This method would be beneficial for applications requiring high resolution SE-fMRI with whole-brain coverage
Introduction
High-spatiotemporal fMRI is difficult to achieve due to long encoding and long TR. Simultaneous Multi-Slice (SMS) has increased temporal efficiency of fMRI1-5, and the faster temporal-sampling has been beneficial to many domains4,6-7. Nonetheless, high multiband (MB) accelerations can lead to high g-factor noise, which potentially limits available TR reduction, particularly for spin-echo (SE) acquisitions. SE-fMRI has been shown to provide improved spatial specificity when compare to GE-fMRI8-10. However, SE-fMRI is technically challenging due to insufficient tSNR&CNR, also high-SAR in SE-SMS which limits TR reduction. Here, we developed ‘complex-basis’ RF-encoding with ‘phase-constrained’ reconstruction to achieve 2× gain in slice-acceleration without temporal smoothing. High-quality SE-fMRI was performed at a combined acceleration of 8-fold using Rinplane×MB×gSlider=2×2×2. SE-fMRI experiment using sensory stimulation was used to demonstrate that doubled slice-acceleration can be achieved with minimal penalty.Methods
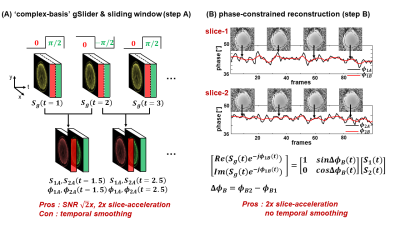
Temporally modulated ‘complex-basis’ gSlider-2 acquisition: Two adjacent slices are acquired together using ‘complex-basis’ RF-encoded slab-encoding, where the excitation phase of the second sub-slice is modulated across time, as shown in Fig.1A, and can be described as:
$$S_{g}(n^{th})=S_{1}(n^{th})e^{j\phi_{1}(n^{th})}+S_{2}(n^{th})e^{j\phi_{2}(n^{th})}e^{j\theta(n^{th})},\begin{cases}\theta(n^{th})=\pi/2 & if\ n^{th} \ is \ odd\\\theta(n^{th})=\ -\pi/2 & if \ n^{th} \ is \ even \end{cases} \ (1)$$
where $$$S_{g}$$$ is gSlider signal from both slices, $$$S_{1}$$$&$$$S_{2}$$$ are signal of slices 1&2, $$$\phi_{1}$$$&$$$\phi_{2}$$$ are the corresponding background phases. $$$\theta(n^{th})$$$ denotes the temporally-modulated RF-encoding phase of slice2 at the nth frame. SLR-based gSlider RFpulse12 was used for 90-excitation, with standard SLR pulse for 180-refocusing. This design resulted in the same 180-peak-voltage and similar 90-peak-voltage as a single-slice acquisition. Note: benefit of complex-valued signal modulation will become clear below.
gSlider reconstruction:
Sliding-window (standard method): Assuming that, $$$S_{1},S_{2},\phi_{1},\phi_{2}$$$ are slowly varying between two time-frames, Eqn.(1) is rewritten as:
$$\begin{cases}S_{g}(t)=S_{1}(t)e^{j\phi_{1}(t)}+jS_{2}(t)e^{j\phi_{2}(t)}\\S_{g}(t+1)=S_{1}(t+1)e^{j\phi_{1}(t+1)}-jS_{2}(t+1)e^{j\phi_{2}(t+1)}\end{cases}\ (2)$$
Correspondingly, signal magnitude and phase of each slice can be obtained using
$$\begin{cases}S_{g}(t)+S_{g}(t+1)\approx 2S_{1}(t)e^{j\phi_{1}(t)}\\S_{g}(t)-S_{g}(t+1)\approx j2S_{2}(t)e^{j\phi_{2}(t)}\end{cases}\ (3)$$
The temporal resolution loss from resolving the slices in this way can be improved by sliding-window reconstruction across TRs (Fig.1A).
Phase-constrained reconstruction (proposed method): Unlike GE-fMRI, the signal change in SE-fMRI is mostly confined to the image magnitude, with little change phase. Moreover, temporal phase variation from physiological noise is also limited. By taking advantage of minimal temporal-phase variations in SE-fMRI, the image background phases reconstructed from the standard method ($$$\phi_{1A},\phi_{2A}$$$, black lines, Fig.1B) were used and averaged across 5 time-frames to remove noise ($$$\phi_{1B},\phi_{2B}$$$, red lines, Fig.1B).This averaged phase was then used as a phase estimate to enable reconstruction of the two imaging slices directly for each acquired time-point, without incurring temporal smoothing. Then, $$$S_{1}$$$&$$$S_{2}$$$ are estimated by inverting the matrix illustrated in Fig.1B.
Visual activation experiment: To compare ‘complex-basis’ gSlider-SMS against standard SMS, data were acquired from two subjects on Siemens Skyra3T with 32-channel coil. In each session, subject was presented with flashing checkboard stimulus (16s-on, 24s-off); each run lasting 184s, with four runs acquired. The following scans were collected, all with TR/TE = 2000/60ms, FOVxy = 220×220mm2, where gSlider-2 provides doubled brain-coverage.
1) 2.5mm isotropic:
i) standard-SMS:Rinplane×MB=2×2,38slices,FOVz=95mm
ii) gSlider-SMS:Rinplane×MB×gSlider=2×2×2,76slices,FOVz=190mm
2) 1.8mm isotropic:
i) standard-SMS: Rinplane×MB=2×2,p.f.7/8,34slices,FOVz=61mm
ii) gSlider-SMS:Rinplane×MB×gSlider=2×2×2,p.f.7/8,76slices,FOVz=122mm
Results
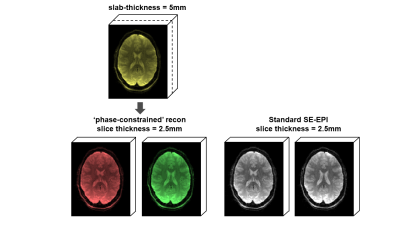
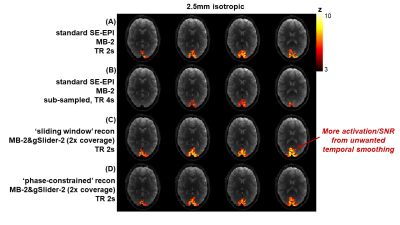
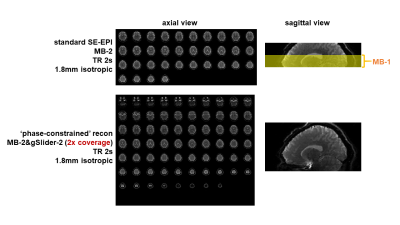
Fig.1B shows spatiotemporal phase during an SE-fMRI acquisition. The phases of adjacent slices are similar, and slowly-varying temporally, making it suitable for the proposed phase-constrained reconstruction. gSlider-2 data with phase-constrained reconstruction are shown in Fig.2, with similar quality to standard single-slice images. Fig.3 shows z-statistic maps (via FSL Feat). As expected, the activation decreased when standard acquisition is sub-sampled to 4s (to mimic acquisition with doubled slice-coverage). The ‘sliding-window’ reconstruction of gSlider-2 data results in stronger activation from noise averaging while also achieving 2× coverage, at a cost of unwanted temporal smoothing. The ‘phase-constrained’ reconstruction maintains high-temporal resolution at 2× brain coverage, and achieves comparable activation to standard acquisition. Fig.4 demonstrates the extended slice-coverage in high-resolution data (1.8mm), where proposed phase-constrained reconstruction exhibits similar quality to standard acquisition, but achieves 2× slice-coverage. Some remaining striping artifacts are observed (sagittally), likely from insufficient 180-crushers in sequence implementation, which is currently being investigated.Discussion and Conclusion
We proposed ‘complex-basis’ gSlider and phase-constrained reconstruction to accelerate SE-SMS-fMRI. The reconstruction relies on spatiotemporal smoothness of image phase in SE-fMRI to enable doubled slice-acceleration with minimal penalty. Derived z-statistics map was shown to be comparable to those from standard SE-SMS-EPI at the same temporal resolution but half the slice-coverage. Although SE-BOLD have lower sensitivity than conventional GE-BOLD13, future work will focus on applying this method to ultra-high field SE-fMRI, where the T2-weighting can enhance microvascular specificity and the higher resolution imaging at ultra-high field will greatly benefit from the 2× increased in slice-acceleration. Application to other time-series data with spatiotemporally smooth phase will also be explored.Acknowledgements
This work was supported in part by NIH research grants: R01EB020613, R01EB019437, R24MH106096, P41EB015896, and the shared instrumentation grants: S10RR023401, S10RR019307, S10RR019254, S10RR023043.References
1. D. J. Larkman, J. V. Hajnal, A. H. Herlihy et al. Use of multicoil arrays for separation of signal from multiple slices simultaneously excited. J. Magn. Reson. Imaging 2001;13(2):313–317.
2. F. A. Breuer, M. Blaimer, R. M. Heidemann et al. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magn. Reson. Med. 2005;53(3):684–691.
3. S. Moeller, E. Yacoub, O. A. Cheryl et al. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain FMRI. Magn. Reson. Med. 2010; 63(5):1144–1153.
4. D. A. Feinberg, S. Moeller, S. M. Stephen et al. Multiplexed echo planar imaging for sub-second whole brain fmri and fast diffusion imaging. PLoS One. 2010; 5(12).
5. K. Setsompop, B. A. Gagoski, J. R. Polimeni et al. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn. Reson. Med. 2012; 67(5): 1210–1224.
6. L. D. Lewis, K. Setsompop, B. R. Rosen et al. Fast fMRI can detect oscillatory neural activity in humans. Proc Natl Acad Sci. 2016;113(43):6679-6685.
7. A. K. Sahib, K. Mathiak, M. Erb et al. Effect of temporal resolution and serial autocorrelations in event-related functional MRI. Magn Reson Med. 2016;76(6):1805-1813.
8. E. Yacoub, T. Q. Duong, P.F. Van De Moortele et al. Spin-echo fMRI in humans using high spatial resolutions and high magnetic fields. Magn. Reson. Med. 2003; 49(4):655–664.
9. N. Harel, J. Lin, S. Moeller et al. Combined imaging-histological study of cortical laminar specificity of fMRI signals. Neuroimage. 2006; 29(3):879–887.
10. S. P. Lee, A. C. Silva, K. Ugurbil et al. Diffusion-Weighted Spin-Echo fMRI at 9 . 4 T : Microvascular / Tissue Contribution to BOLD Signal Changes. Magn. Reson. Med. 1999; 928(5):919–928.
11. K. Setsompop, Q. Fan, J, Stockmann et al. High-resolution in vivo diffusion imaging of the human brain with generalized slice dithered enhanced resolution: Simultaneous multislice (gSlider-SMS). Magn. Reson. Med. 2017;0(9):1–11.
12. Ma J., T. Witzel, W.A. Grissom et al. Minimum peak power root-flipped gSlider-SMS RF pulses for high-resolution in vivo diffusion imaging. ISMRM. 2017, page 523.
13. K. Boyacioǧlu, J. Schulz, N. C. J. Müller et al. Whole brain, high resolution multiband spin-echo EPI fMRI at 7T: A comparison with gradient-echo EPI using a color-word Stroop task. Neuroimage. 2014;97:142-150.
Figures