3405
Development of a 24-Channel 3T Multi-Array Coil for functional MRI in awake monkeysAtsushi Yoshida1, Yoki Hori1, Kantaro Nishigori1,2, Masahiro Ohno1, Yoshihiko Kawabata3, Yuta Urushibata4, Katsutoshi Murata4, Masataka Yamaguchi1, Joonas Autio1, Matthew F Glasser5,6, and Takuya Hayashi1
1Center for Life Science Technologies, RIKEN, Kobe, Japan, 2Sumitomo Dainippon Pharma Co. Ltd, Osaka, Japan, 3Takashima Seisakusho Co. Ltd., Tokyo, Japan, 4Siemens Healthcare Japan, Tokyo, Japan, 5Department of Anatomy and Neurobiology, Washington University Medical School, St. Louis, MO, United States, 6St. Luke's Hospital, St. Louis, MO, United States
Synopsis
High-resolution fMRI in awake macaques may address compelling questions for how the brain is dynamically organized to create behaviors. Here, we developed a 24-channel multi-array receive coil for awake macaques and a 3T MRI scanner. High performance of the coil was confirmed by assessing noise correlation, B0/B1 field and SNR. Preliminary resting-state fMRI data, preprocessed with the Human Connectome Project pipeline, revealed a number of functional network components, some of which replicated previous findings. Our system may be useful for multi-modal cortical mapping of task-dependent and resting functional activity.
Introduction
The macaque monkey is an important animal model for neuroscience. MR imaging techniques such as functional MRI (fMRI) and diffusion MRI (dMRI) have provided valuable insights for understanding brain organization1,2,3, as well as cross-species anatomy4,5. Recent advances in high-temporal resolution fMRI6,7 have facilitated artifact removal8 and sensitive detection of complex functional networks9. We previously developed a 24-channnel multi-array receive coil for anesthetized macaque imaging in a 3T MRI scanner, and confirmed its usefulness for fast imaging to explore and validate non-invasive connectivity measures10. However, there is a compelling need to address brain organization in conscious behaving animals. Here, we developed a separate 24-channel multi-array coil optimized for headpost-implanted monkeys and evaluated measures of coil quality and efficiency in phantoms and animal studies.Methods
Animal experiments were approved by the Animal Care and Use Committee of the Kobe Institute of RIKEN. The subject was a male rhesus monkey (Macaca mulatta; 6.5 kg). Experiments were performed in a 3T MRI scanner with a powerful gradient (80mT/m) (MAGNETOM Prisma, Siemens, Erlangen, Germany). The coil frame geometry was designed with a 3D digital design software (Rhinoceros5, McNeel, Seattle, USA) to closely fit our largest monkey’s head. The coil elements covered the whole monkey’s head and were placed with some overlap with each other to avoid coupling. To easily equip the coil over the headpost-implanted monkey, the coil was separated into anterior and posterior halves (Figure 1A), and the coil elements between two halves were overlapped by bending a part of elements outward and facing each other (Figure 1B). Element coupling was estimated with noise correlation measurements. B1 transmission (B1+) was assessed with a flip-angle (FA) sequence. B1 receive field (B1-) was estimated by calculating signal ratio between 24-channel and body receive coils with gradient-echo sequence. To evaluate the quality of functional MR images without the additional confounds of awake imaging such as motion, we scanned resting-state fMRI (rfMRI) using imaging parameters in one anesthetized animal (FOV 95x95 mm, matrix size 76x76, slice thickness 1.25 mm, multiband factor=5, TR=755ms, TE 30ms, number of slices 50, flip-angle 55°, bandwidth 1370 Hz/pixel, echo spacing 0.95ms, the number of total volumes 4096, and scan time 51 min). Data was analyzed with non-human primate version of Human Connectome Project (HCP) pipelines11. The fMRI data was analyzed with a single-session independent component analysis (ICA) and manually surveyed for structured noise and neural components. T1w and T2w images were acquired as described in our other abstract10.Results
The element decoupling was verified by measuring the noise correlation coefficient matrix for the 24-channel coil (Figure 1C). The average noise correlation coefficient was 8.5±5.3%. B0 field-maps illustrated inhomogeneities near nasal and mastoid cavities (Figure 2A). Flip-angle (B1+) maps showed that the transmission was slightly higher at subcortical regions but fairly uniform over the cortical surface (89°±4.5°) (Figure 2B), and B1 receive map (B1-) also showed signal sensitivity is higher in the peripheral part of the brain, particularly at the gyrus crown located close to the coil elements (Figure 2C). The B1 bias correction using T1w and T2w worked fine with the HCP pipeline, and myelin map was created as in Figure 2D. ICA of a single resting state fMRI scan showed 231 structured noise components and 19 neural signal components. There was no structured noise related to slice overlapping. Figure 3 shows representative resting-state network (RSN) components (default mode, frontoparietal, ventral sensorimotor, and auditory networks).Discussion
Our new system with a 24-ch multi-array coil for awake macaques in a 3T scanner showed promising coil performance. Although the coil was separated in two units of for usability, the coupling of elements was minimized and average value was almost same as in our previous single-unit 24-ch coil for anesthetized macaques10. The B1 transmission field was fairly homogenous over the cortical surface. B1 receive field was sensitive to the superficial part of the brains such as gyral crowns, however, the biasfield was well corrected within the HCP pipeline using T1w and T2w, and resulted in myelin maps consistent with previous reports in macaque12. The coil detected a number of RSNs, some of which were consistent with our other report10. Overall findings suggest the potential of our system, although we need to test it in awake animals with a focus on head motion and the tolerance of the animals.Conclusion
We developed a system with 24-ch multi-array coil for awake macaques in a 3T MRI scanner. Our preliminary data confirmed that coil performance was high enough for multi-modal imaging in awake monkeys.Acknowledgements
This research is partially supported by the program for Brain Mapping by integrated Neurotechnologies for Disease Studies (Brain/MINDS) from Japan Agency for Medical Research and development, AMED, by MEXT KAKENHI Grant (17K01981, 16H03300, 16H01626), and by a grant from Sumitomo Dainippon Pharma Co., Ltd.References
[1]. Saleem KS, et al., Magnetic resonance imaging of neuronal connections in the macaque monkey. Neuron. 2002;34;685-700. [2]. Logothetis NK, et al., Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412;150-157. [3]. Donahue CJ, et al., Using Diffusion Tractography to Predict Cortical Connection Strength and Distance: A Quantitative Comparison with Trace in the Monkey. J Neurosci. 2016;36;6857-6870. [4]. Nakahara K. et al., Functional MRI of macaque monkeys performing a cognitive set-shifting task. Science. 2002;295;1532-1536. [5]. Mantini D. et al., Interspecies activity correlations reveal functional correspondence between monkey and human brain areas. Nat Methods. 2012;9;277-282. [6]. Ugurbil K, et al. Pushing spatial and temporal resolution for functional and diffusion MRI in the Human Connectome Project. NeuroImage. 2013;80-104. [7]. Moeller S, et al. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med. 2010;63;1144-1153. [8]. Griffanti L, et al. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. NeuroImage. 2014;95;232-247. [9]. Smith SM. et al., Temporally-independent functional modes of spontaneous brain activity. Proc Natl Acad Sci U S A. 2012;109;3131-3136. [10]. Joonas A. et al., Macaque connectome using a 24 channel multi-array coil in 3T MRI scanner. The 40th Annual Meeting of the Japan Neuroscience Society. Abstract 1P-387. [11]. Glasser MF, et al. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage. 2013;80;105-124. [12]. Glasser MF. et al., Trens and properties of human cerebral cortex: correlations with cortical myelin content. NeuroImage. 2014;93;165-175.Figures
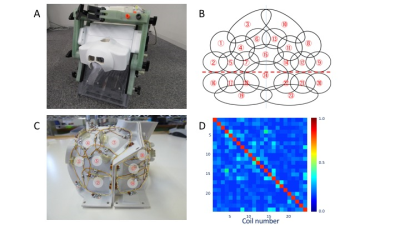
Fig1
(A) Appearance of the coil. (B) Coil element arrangement and labeling flattened
into a 2D representation. The coil is separated into anterior and posterior
halves along horizontal red dot line. (C) Coil with element arrangements. (D)
Noise correlation matrix.
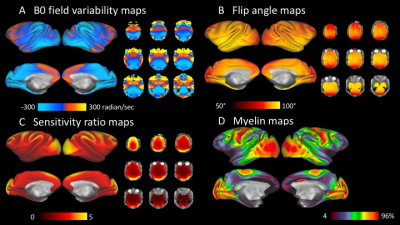
Fig2
Data quality assessment in vivo.
Surface
and volume maps of B0 field (A), flip angle (B) and sensitivity ratio (C). (D)
Myelin surface maps.
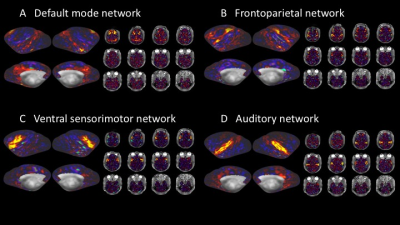
Fig3
Representative resting-state networks.
(A)
Default mode network, (B) Frontoparietal network, (C) Ventral sensorimotor
network, and (D) Auditory network.