3389
Empirical Sequence Design for Combined T2-Preparation and Outer Volume Suppression Preparation Sequence1Electrical Engineering, Stanford University, Stanford, CA, United States, 2Medical Biophysics, Robarts Research Institute, London, ON, Canada, 3Cardiovascular Medicine, Stanford University, Stanford, CA, United States, 4Cardiology, Palo Alto Medical Foundation, Palo Alto, CA, United States
Synopsis
A new combined T2-prepared, multidimensional OVS pulse sequence was designed by an empirically-driven method. We defined a T2-prepared OVS module as a tip-down pulse, refocusing sequence, and a selective tip-up pulse. Candidate pulses were proposed for each portion of the module and all possible modules were evaluated by a metric based on Bloch simulations. Multidimensional OVS was achieved by concatenation of modules. The proposed sequence had the lowest metric and was compared against an existing T2-prepared OVS sequence for in vivo (n=5) coronary imaging. The proposed sequence had superior vessel sharpness, SNR, OVS, and qualitative reader scores.
Introduction
Outer volume suppression (OVS) with T2-preparation for coronary magnetic resonance angiography reduces FOV to shorten scan time and helps suppress aliasing and motion artifacts to improve image quality1. An empirically-driven, combinatorial search of potential sequences was used to create a new combined T2-prepared, multidimensional OVS pulse sequence with adaptable spectral and spatial selectivity, robustness to B0 and B1 inhomogeneities, and low SAR.Methods
Sequence Design
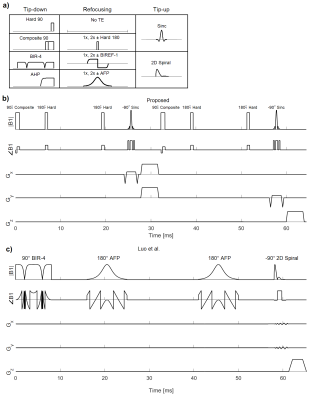
We defined a module of T2-prepared OVS as a non-selective tip-down pulse, a refocusing sequence, and a selective tip-up pulse. The candidates for each pulse are shown in Figure 1a. The module is reversible such that the selective pulse is first. We concatenated two modules to achieve 2D OVS, including with spiral tip-up.
Bloch simulations calculated the resultant longitudinal magnetization for each candidate sequence. Passband and stopband performance were evaluated along both the x-axis and y-axis to account for differences from suppressing one dimension at a time. To evaluate off-resonance and B1 robustness, the candidates were evaluated at [-64,0,+64] Hz and [80%, 100%, 120%] B1. Candidates were evaluated based upon an L1-metric of passband distance from unity and stopband distance from zero. In the initial search, the candidates were simulated at only three points of off-resonance and B1 inhomogeneity as a tradeoff to efficiently search the combinatorially large parameter space. Similarly, the refocusing pulses were limited to a maximum of two pulses to limit SAR, complexity, and scope.
The top-performing candidates were more thoroughly simulated across B0 and B1 inhomogeneities with the same metric. While the L1-metric was the primary discriminant, SAR and spatial and spectral flexibility were also qualitatively considered. The SAR consideration diminished considerations for sequences with similar performance but adiabatic pulses. The flexibility consideration gave greater consideration for sequences with sinc tip-ups. The chosen candidate module is a 90°-60180°60 composite pulse2, two positive 180°Y hard pulses, and a -90° sinc tip-up (Figure 1b).
Imaging Experiments
Among several existing T2-prepared, 2D OVS sequences, we chose to compare against the sequence of Luo et al. which uses a robust BIR-4 tip-down, paired adiabatic refocusing, and a 2D tip-up (Figure 1c)1,3. All experiments were conducted on a 1.5 T GE scanner with an 8-channel cardiac receive coil, following institutional review board guidelines. Phantom experiments were acquired with 2D gradient-echo to evaluate off-resonance and B1 robustness. Experiments were performed at [-128, -64, 0, +64, +128] Hz and [80%,100%,120%] B1. Coronary images were acquired on five healthy volunteers with an alternating-TR steady-state free precession 3D-cones phyllotaxis trajectory with 3D-iNAVs and translational motion correction4,5. Coronary images used a spectral-spatial sinc tip-up with fat placed in the stopband to leave fat saturated6. The Luo sequence did not have fat saturation.
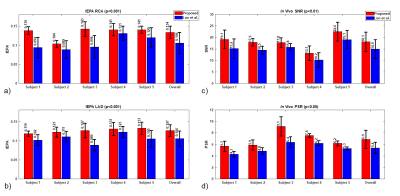
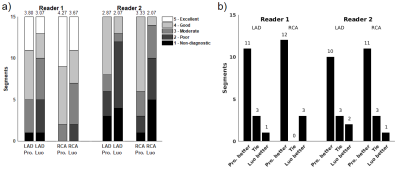
To evaluate image quality, signal-to-noise ratio (SNR) divided blood pool signal with background standard deviation. To evaluate OVS sequence suppression, passband-to-stopband ratio (PSR) divided passband myocardium signal with stopband chest wall skeletal muscle signal. Image edge profile acutance (IEPA) was used to evaluate coronary sharpness7. Two board-certified cardiologists evaluated coronary images in a double-blind reading for both sequences on a five-point scale: 5-Excellent, 4-Good, 3-Moderate, 2-Poor, 1-Non-diagnostic. Significance was determined by two-tailed Student’s t-test8. Readers also gave rank scores to show preference between the two images in a pair. Significance was determined by Wilcoxon signed-rank tests8.
Results and Discussion
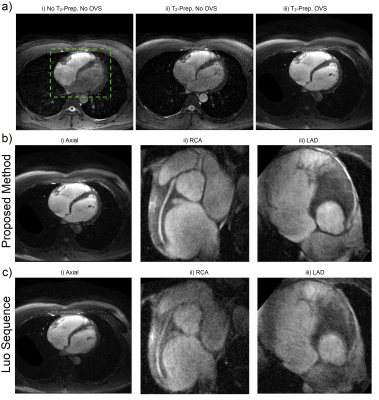
Figure 2 shows comparison phantom results with cross-sectional plots of the proposed sequence and Luo sequence. The top row shows that suppression in the tip-up bands of each OVS module is robust to inhomogeneities. The four corners which are never tipped-up have worse suppression with respect to inhomogeneities because their suppression is based entirely upon an assumed 90° tip-down and subsequent spoiling. The results at +128 Hz are noticeably worse because of the composite tip-down spectral response. This was addressed in the coronary experiments by using a spectral-spatial tip-up which leaves isochromats outside the spectral passband saturated, alleviating off-resonance robustness requirements and reducing off-resonance blurring. The top row also has sharper transition bands than the bottom row from using high time-bandwidth sincs, compared to 2D spirals.
Figure 3 shows sample comparison images of axial and coronary views from each pulse sequence. IEPA, SNR, and PSR results are summarized in Figure 4 with the proposed sequence outperforming the Luo sequence in all subjects. Reader scores and comparisons are shown in Figure 5 and the proposed sequence is superior in both.
The proposed sequence can achieve a uniform, high time-bandwidth product passband, leading to better intensity-based motion correction algorithm performance and thus potentially contributing to image quality9. The proposed sequence also has 48% less SAR than the Luo sequence.
Acknowledgements
This research is supported by NIH R01 HL127039, GE Healthcare, Joseph W. and Hon Mai Goodman Stanford Graduate Fellowship, and the National Science Foundation Graduate Research Fellowship under Grant No. DGE-114747.References
1. Luo J, et al. Combined Outer Volume Suppression and T2 Preparation Sequence for Coronary Angiography. Magn Reson Med. 2015;74(6):1632-1639.
2. Levitt MH. Symmetrical composite pulse sequences for NMR population inversion. I. Compensation of radiofrequency field inhomogeneity. J Magn Reson. 1969;48(2):234-264.
3. Coristine AJ, van Heeswijk RB, Stuber M. Combined T2-preparation and two-dimensional pencil-beam inner volume selection. Magn Reson Med. 2015;74(2):529-536.
4. Addy NO, Ingle RR, Wu HH, Hu BS, Nishimura DG. High-resolution variable-density 3D cones coronary MRA. Magn Reson Med. 2015;74(3):614-621.
5. Malave MO, Addy NO, Ingle RR, Cheng, JY, Baron CA, Nishimura DG. Analysis of Motion and Eddy Currents with 3D Cones Reordering for Whole-Heart Coronary MR Angiography. Proc ISMRM 24, Singapore, 2016:1852.
6. Meyer C, Pauly J, Macovski A, Nishimura DG. Simultaneous spatial and spectral selective excitation. Magn Reson Med. 1990;15(2):287-304.
7. Delgado-Olabarriaga S, Rangayyan RM. Subjective and objective evaluation of image sharpness: behavior of the region-based image edge profile acutance measure. Proc SPIE Medical Imaging. 1996;2712.
8. McDonald J, Handbook of Biological Statistics. Baltimore: Sparky House Publishing; 2009.
9. Wu W, Koopmans PJ, Frost R, Miller KL. Reducing slab boundary artifacts in three-dimensional multislab diffusion MRI using nonlinear inversion for slab profile encoding (NPEN). Magn Reson Med. 2016;76(4):1183-1195.
10. de Graaf RA, Luo Y, Terpstra M, Merkle H, Garwood M. A new localization method using an adiabatic pulse, BIR-4. J Magn Reson B. 1995;106(3):245-252.
11. Ugurbil K, Garwood M, Rath AR, Bendall MR. Amplitude- and frequency/phase-modulated refocusing pulses that induce plane rotations even in the presence of inhomogeneous B1 fields. J Magn Reson. 1988;78(3):472-497.
12. Pauly J, Nishimura DG, Macovski A. A k-space analysis of small-tip-angle excitation. J Magn Reson. 1989;81(1):43-56.
Figures