3351
Improved Golden Ratio Radial Arterial Spin Labeling Angiography Reconstruction using k-t Sparsity Constraints1Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom
Synopsis
Dynamic arterial spin labeling angiography enables non-invasive visualization of arterial flow patterns, but is often time-consuming to perform. Undersampled radial trajectories help reduce acquisition time, but can result in noise-like aliasing artefacts and reduced spatial fidelity, particularly for a combined angiographic and perfusion golden ratio imaging technique, CAPRIA. An image reconstruction framework leveraging coil information and sparsity in the spatial and temporal frequency domains is presented which reduces aliasing and improves image sharpness in both 2D and 3D data. In addition, scan time reductions up to 10x are shown to be feasible whilst maintaining spatial and temporal information.
Introduction
Time-resolved arterial spin labeling (ASL) angiography allows for non-invasive visualization of arterial structures and hemodynamics1,2, valuable in cerebrovascular disease. While whole-brain dynamic 3D data is time-consuming to acquire, radial trajectories allow significant undersampling with relatively benign artefacts in the sparse angiographic data after subtraction of label and control images3–5, helping reduce scan times, but residual aliasing could still obscure smaller vessels. This is particularly problematic for the recently introduced combined angiography and perfusion using radial imaging and ASL (CAPRIA) sequence6,7, which provides simultaneous visualization of arterial blood flow and tissue perfusion. Extra care must be taken to limit ASL signal attenuation prior to accumulation in the tissue by keeping excitation flip angles small and the repetition time relatively long, leading to low signal strength and high undersampling factors, which also causes blurring through reduced sampling of high spatial frequencies.
Here, we optimize the reconstruction of CAPRIA angiographic images utilizing coil information and compressed sensing, leveraging the considerable sparsity present in both the spatial and temporal frequency domains. We demonstrate the significant potential for improved image quality and scan time reduction with this approach.
Methods
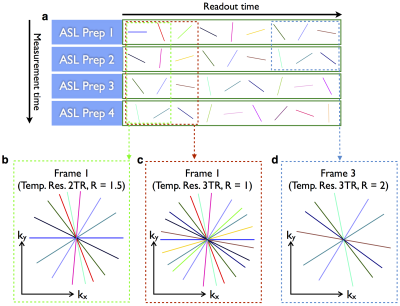
A 2D CAPRIA acquisition schematic is shown in Figure 1, although the concept applies equally in 3D. With this flexible golden ratio approach, images can be reconstructed at arbitrary temporal resolution, chosen retrospectively (Figure 1b,c). In addition, we can test the image quality that would be obtained in a shorter scan by discarding data acquired during part of the acquisition (Figure 1d).
The proposed reconstruction solves for a time-series $$$x$$$ of images that is sparse in the x-f domain:
$$ ||F_xSx-d||^2_{2}+\lambda||F_tx||_1$$
where $$$F_x$$$ is an under-sampled non-uniform Fourier transform in space, $$$S$$$ is a sensitivity encoding operator, $$$d$$$ is the complex subtracted (control – tag) k-space data, and $$$F_t$$$ is the temporal Fourier transform. The optimization was solved using FISTA8 with a fixed step size of 0.5, and $$$\lambda$$$= $$$2x10^{-6}$$$/$$$1x10^{-8}$$$ for the 2D/3D reconstructions respectively.
Sensitivities were estimated from data averaged across tag/control conditions and all time points using the adaptive combine method9 . For robust complex subtraction, global phase differences between the tag and control data were corrected by fitting each control k-space line to the corresponding tag line, and removing their phase offset.
This approach was evaluated against a previously used6,7 coil-by-coil regridding algorithm10 in 2D and 3D CAPRIA 3T data with pseudocontinuous ASL labeling duration 1.4s, readout duration 2s and the following imaging parameters: 2D data6 - four volunteers, 1.1x1.1x10mm voxels, TR/TE 12/6ms, 7° flip angle, 4 slices, 2.5min per slice; 3D data7 – four volunteers, 1.1x1.1x1.1mm voxels, TR/TE 9/3.4ms, 6° flip angle, 10min acquisition.
Results
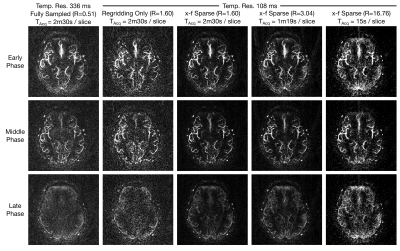
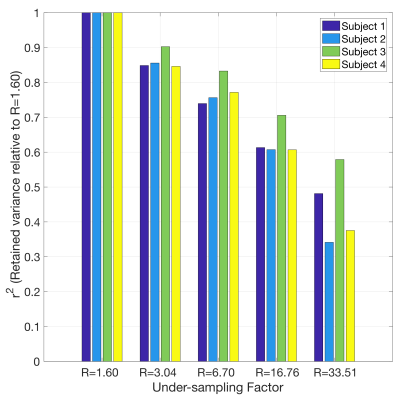
Figure 2 shows 2D reconstructions in a representative subject. The x-f sparse reconstructions remove much of the noise-like aliasing whilst retaining the spatial and temporal characteristics of the angiographic images, even at higher under-sampling factors, corresponding to considerably reduced scan times (15s per slice). Figure 3 plots the retained variance in the x-f reconstructions across under-sampling factors and subjects, relative to the least under-sampled case (R=1.60). Using less than 25% of the data at R=6.70 still results in r2>0.7 across all subjects.
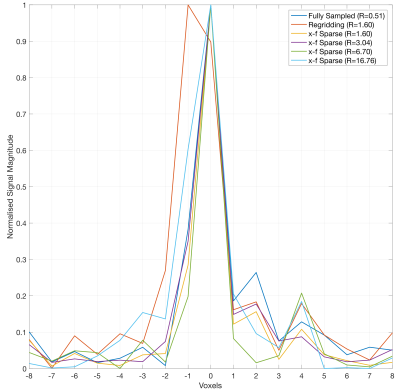
To assess spatial degradation, Figure 4 shows signal profiles across a middle cerebral artery branch. The x-f reconstructions at R=6.70 and below are indistinguishable from the fully sampled reference, whereas slight blurring is apparent at R=16.76. In comparison, the considerably blurred regridded image almost doubles the FWHM of the vessel profile.
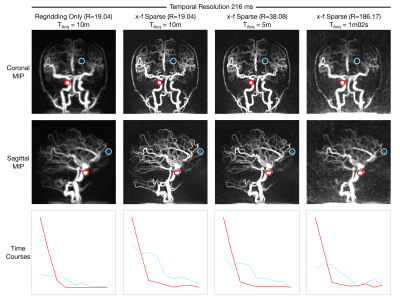
Figure 5 shows maximum intensity projections and example time-courses from the 3D reconstructions. The improved sharpness of the x-f reconstructions compared to regridding is apparent, even at higher under-sampling factors. The R=186.17 data can be acquired in 10x less time than the reference R=19.04 data, nearly one minute, while preserving most of the spatial and temporal features in the regridded images.
Discussion
We have presented an improved reconstruction technique for CAPRIA angiographic images which considerably reduced aliasing and improved image sharpness, permitting high undersampling factors whilst retaining image fidelity. Due to the nature of the golden ratio CAPRIA acquisition, higher undersampling reconstructions correspond exactly to data acquired with shorter acquisition durations, demonstrating the feasibility of 1-5min 3D acquisitions, improving clinically applicability. The proposed reconstruction should also work equally well for other ASL angiographic acquisitions.
In further work, we aim to more rigorously evaluate the proposed reconstruction technique and its preservation of timing information at higher undersampling factors. The next challenge is to extend this framework to reconstruct perfusion images from the same raw data, as shown previously6,7, whilst accommodating the reduced spatial sparsity of the perfusion signal.
Acknowledgements
M.C. and T.W.O. are supported by the UK Royal Academy of EngineeringReferences
1. Bi X, Weale P, Schmitt P, Zuehlsdorff S, Jerecic R. Non-contrast-enhanced four-dimensional (4D) intracranial MR angiography: A feasibility study. Magn Reson Med 2010; 63: 835–841.
2. Sallustio F, Kern R, Gunther M, Szabo K, Griebe M, Meairs S et al. Assessment of Intracranial Collateral Flow by Using Dynamic Arterial Spin Labeling MRA and Transcranial Color-Coded Duplex Ultrasound. Stroke 2008; 39: 1894–1897.
3. Wu H, Block WF, Turski PA, Mistretta CA, Johnson KM. Noncontrast-enhanced three-dimensional (3D) intracranial MR angiography using pseudocontinuous arterial spin labeling and accelerated 3D radial acquisition. Magn Reson Med 2013; 69: 708–715.
4. Wu H, Block WF, Turski PA, Mistretta CA, Rusinak DJ, Wu Y et al. Noncontrast dynamic 3D intracranial MR angiography using pseudo-continuous arterial spin labeling (PCASL) and accelerated 3D radial acquisition. J Magn Reson Imaging 2014; 39: 1320–1326.
5. Koktzoglou I, Meyer JR, Ankenbrandt WJ, Giri S, Piccini D, Zenge MO et al. Nonenhanced arterial spin labeled carotid MR angiography using three-dimensional radial balanced steady-state free precession imaging. J Magn Reson Imaging 2015; 41: 1150–1156.
6. Okell TW. Combined Angiography and Perfusion using Radial Imaging and Arterial Spin Labeling. In: Proceedings 24th Scientific Meeting, ISMRM. Singapore, 2016, p 1001.
7. Okell TW. 4D Combined Angiography and Perfusion using Radial Imaging and Arterial Spin Labeling. In: Proceedings 25th Scientific Meeting, ISMRM. Hawaii, USA, 2017, p 675.
8. Beck A, Teboulle M. A Fast Iterative Shrinkage-Thresholding Algorithm for Linear Inverse Problems. SIAM J Imaging Sci 2009; 2: 183–202.
9. Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. Magn Reson Med 2000; 43: 682–90.
10. Fessler JA, Sutton BP. Nonuniform fast fourier transforms using min-max interpolation. IEEE Trans Signal Process 2003; 51: 560–574.
Figures