3347
Rapid, non-contrast thoracic MRA using a combination of stack-of-star k-space sampling, compressed sensing, and self-navigation of respiratory motion1Biomedical Engineering, Northwestern University, Evanston, IL, United States, 2Radiology, Northwestern University, Chicago, IL, United States, 3Division of Pediatric Cardiology, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States, 4Department of Medical Imaging, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States, 5Radiology, Northwestern University, Feinberg School of Medicine, Chicago, IL, United States
Synopsis
We sought to highly accelerate high resolution (1.5 mm x 1.5 mm x 1.5 mm) non-contrast thoracic MRA using a combination of compressed sensing, stack-of-stars k-space sampling with variable density, and self-navigation , and we compared its performance against clinical contrast-enhanced MRA in patients with suspected aortic disease.
Introduction
Thoracic aortic aneurysms (TAAs), while rare, are life-threatening conditions which require routine1 monitoring with imaging to determine when surgical correction is needed (e.g., aortic diameter >5 cm in adults2) Contrast-enhanced magnetic resonance angiography (CE-MRA) is routinely used for evaluation of aortic disease, but requires gadolinium-based contrast agent (GBCA). The reliance on GBCA for CE-MRA is troubling given the recent evidence of GBCA deposits in the brain after repeated exposure3. A previous study has reported the clinical utility of non-contrast thoracic MRA (NC-MRA) using a navigator-gated, balanced steady-state free precession (b-SSFP) pulse sequence with a spatial resolution of 1.5 mm x1.5 mm x 3 mm and a scan time on the order of 6-10 min4. For clinical translation, it may be necessary to increase the slice resolution and reduce the scan time. In this study, we sought to highly accelerate NC-MRA using a combination of compressed sensing (CS), stack-of-stars k-space sampling with variable density, and self-navigation of respiratory motion5 to increase the spatial resolution (from 3 to 1.5 mm slice thickness) and reduce the scan time (from ~8 to 5 min), and compare its performance against clinical CE-MRA in patients with suspected aortic disease.Method
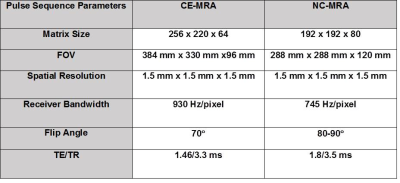
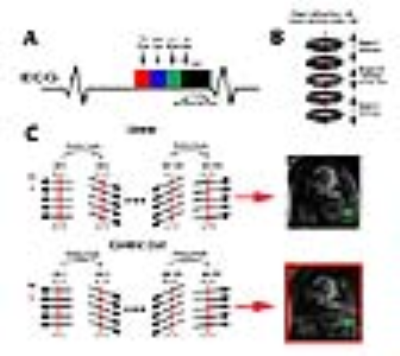
(Patients) We scanned 6 patients (4 males, age= 58 ± 5.7 years) who were undergoing a clinical MRA with standard dose of GBCA on a 1.5T scanner (Siemens, Avanto), where NC-MRA was performed prior to administration of GBCA. (Pulse Sequence) We modified a 3D b-SSFP pulse sequence to employ a stack-of-star sampling pattern with variable density that oversampled the central partitions of k-space, as previously described6 , and one ray per heart-beat was oriented along the head-to-foot direction to track the respiratory motion, as previously described5. As shown in figure 1, we explored two different k-space ordering along kz: (a) linear and (b) “centric” out. We elected to use a kz ordering that produced better fat suppression in preliminary experiments (Figure 1C). NC-MRA utilized a T2 preparation time = 50 ms, fat saturation, α/2 preparation pulse, and 28,000 rays to reconstruct 6 respiratory phases (Figure 2). The performance of NC-MRA was compared with clinical steady-state CE-MRA7, which was performed following GBCA administration ranging from 0.15 – 0.20 mmol/kg and inversion recovery time (TI) = 260 ms and b-SFFP readout with navigator gating. Relevant imaging parameters for both NC-MRA and CE-MRA are described in table 1. (Image Reconstruction) The CS8 reconstruction was performed off-line on a workstation equipped with MATLAB (R2014a, The MathWorks). After nonuniform fast Fourier transform9 and self-calibration of coil sensitivity maps10, a CS reconstruction using 60 iterations was performed with total variation along the respiratory motion and slice dimensions with normalized weight of α=0.00075 and β=0.00015, respectively, and normalized fidelity weight of 0.006(Figure 2)11. (Image Quality) One radiologist and one cardiologist visually graded the scores on a 5-point scale (1: worst; 3: clinically acceptable; 5: best) for 3 categories: conspicuity of lumen wall, artifact, and noise. The mean reader scores were compared using the Wilcoxon signed-rank test, where p<0.05 was considered significant. (Vessel Diameters) Another reader measured the vessel diameters at nine standard locations12. The vessel diameters were compared using a two tailed paired t-test and the Bland-Altman (BA) and linear regression analyses.Results
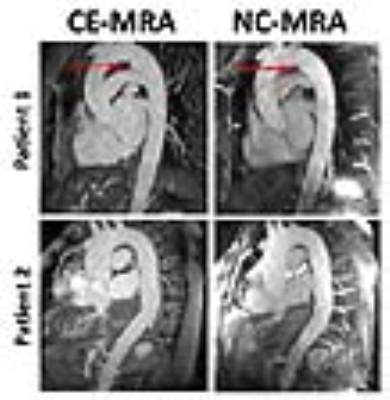
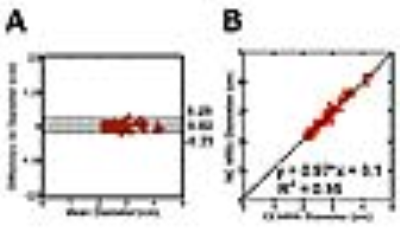
The mean scan time for clinical CE-MRA and NC-MRA was 5:54 ± 2:37 and 5:47 ± 0:48 min, respectively. Figure 3 shows representative reformatted maximum-intensity-projection (MIP)s of two patients where in one patient an aneurysm is clearly depicted in both CE-MRA and NC-MRA. Averaging the results over 6 patients, the conspicuity (4.7 ± 0.5 for CE-MRA; 4.6 ± 0.4 for NC-MRA), artifact (3.9 ± 0.5 for CE-MRA; 3.9 ± 0.6 for NC-MRA), and noise (4.6 ± 0.5 for CE-MRA; 4.2 ± 0.5 for NC-MRA) scores were not significantly different (p>0.05), and all three scores were greater than 3.0 (clinically acceptable). In addition, the vessel diameters were not significantly different (p>0.05) between MRAs. Figure 4 shows the scatter plots resulting from the Bland-Altman and linear regression analyses, indicating that the vessel diameters are strongly correlated and in good agreement.Conclusion
This study describes development and evaluation a 3D NC-MRA pulse sequence using a combination of stack-of-star k-space sampling, self-navigation of respiratory motion, and CS. In six patients, the proposed NC-MRA produced reader scores and aortic diameters that were not significantly different from those produced by CE-MRA. Future studies in a larger patient cohort are warranted to further evaluate the clinical utility.Acknowledgements
NIH grant: R01HL116895, R01HL138578, R21EB024315, R21AG055954References
1. Pearson GD, Devereux R, Loeys B, et al. Report of the national heart, lung, and blood institute and national marfan foundation working group on research in marfan syndrome and related disorders. Circulation. 2008;118(7):785-91.
2. Gersh BJ, Maron BJ, Bonow RO,et al. American Association for Thoracic Surgery; American Society of Echocardiography; American Society of Nuclear Cardiology. Heart Failure Society of America. 2011:2761-96.
3. Olchowy C, Cebulski K, Łasecki M, et al. The presence of the gadolinium-based contrast agent depositions in the brain and symptoms of gadolinium neurotoxicity-A systematic review. PloS one. 2017;12(2).
4. Amano Y, Takahama K, Kumita S. Non–contrast‐enhanced MR angiography of the thoracic aorta using cardiac and navigator‐gated magnetization‐prepared three‐dimensional steady‐state free precession. JMRI. 2008;27(3):504-9.
5. Feng L, Axel L, Chandarana H, Block KT, et al. XD‐GRASP: Golden‐angle radial MRI with reconstruction of extra motion‐state dimensions using compressed sensing. MRM. 2016; 75(2):775-88. 6. Bhat H, Yang Q, Zuehlsdorff S, et al. Contrast‐enhanced whole‐heart coronary magnetic resonance angiography at 3 T with radial EPI. MRM. 2011 Jul 1;66(1):82-91.
7. Galizia MS, Febbo JA, Popescu AR, et al. Steady state imaging of the thoracic vasculature using inversion recovery FLASH and SSFP with a blood pool contrast agent. JCMR. 2012 ;14(S1):P51.
8. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. MRM. 2007 ;58(6):1182-95.
9. Fessler JA. On NUFFT-based gridding for non-Cartesian MRI. Journal of Magnetic Resonance. 2007;188(2):191-5.
10. Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. MRM. 2000;43(5):682-690.
11. Piccini D, Feng L, Bonanno G, et al. Four‐dimensional respiratory motion‐resolved whole heart coronary MR angiography. MRM. 2017;77(4):1473-84.
12. Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Journal of the American College of Cardiology. 2010;55(14):e27-129.
Figures