3344
MRI and Non-Contrast CT Image Fusion to Guide Vascular Intervention: Feasibility Using Ferumoxytol in Patients with Renal Failure1Diagnostic Cardiovascular Imaging Laboratory, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States, 2Department of Radiology, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States, 3Division of Cardiology, David Geffen School of Medicine at UCLA and VA Greater Los Angeles Healthcare System, Los Angeles, CA, United States
Synopsis
Accurate pre-procedural vascular mapping may be crucial to guide successful intervention. Whereas MRA provides excellent definition of the perfused vascular lumen, it is insensitive to vascular calcification and may fail to image indwelling devices. CTA can address the latter limitations, but may be contraindicated in patients with renal impairment, as is the case for gadolinium based contrast agents (GBCA). Our early results suggest that, in patients with renal failure, 3D fusion of non-contrast CT and ferumoxytol-enhanced MR images leverages the complementary strengths of both modalities while avoiding both iodinated contrast agents and GBCA.
INTRODUCTION
Whereas magnetic resonance angiography (MRA) provides excellent intraluminal contrast, it is insensitive to vascular calcification and fails to image indwelling metal devices due to artifact1. Computed tomography (CT) is exquisitely sensitive to calcium and can image a variety of indwelling hardware devices. However, CT angiography requires injection of iodinated contrast agents, which are generally avoided in renal impairment due to the attendant risk of nephrotoxicity. Similar concerns about nephrogenic systemic fibrosis limit the use of GBCA in this patient population. We hypothesize that in patients where ferumoxytol is a suitable alternative to CT angiography2,3, calcification and hardware devices may be accurately registered to vascular luminal anatomy by fusing non-contrast CT and ferumoxytol enhanced MRA (FE-MRA) images. In a proof-of-concept study, we aim to evaluate the feasibility of FE-MRA and non-contrast CT image fusion for pre-procedural vascular assessment of patients with renal impairment.METHODS
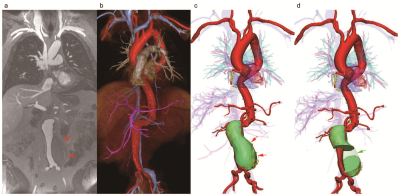
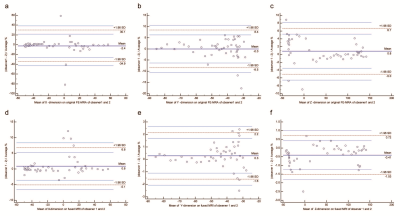
This is an IRB-approved and HIPAA-compliant study. Fifteen consecutive patients who had both FE-MRA and non-contrast CT performed between January 2015 to August 2017 were selected for analysis. 3D MR and CT images were segmented and registered using Mimics (Materialise®, Leuven, Belgium). Unambiguous landmarks such as implantable medical devices were defined on 3D models of both modalities, followed by semi-automatic registration for fusion and manual adjustment as needed. In a subgroup of 5 patients, a total of 247 regions of interest (ROIs) were marked on implantable medical devices in both the original FE-MRA and fused FE-MRA using a DICOM viewing software (OsiriX®, Geneva, Switzerland). The deviation lengths between the corresponding ROIs of each dataset were used to evaluate the accuracy of image registration. Two observers independently performed image registration on one patient (47 ROIs) and intraclass correlation coefficients (ICCs) were used to determine interobserver agreement.RESULTS
Fifteen patients (age 76±12 years, 7 female) with aortic stenosis (n=13) or aortic aneurysm (n=2) and chronic kidney disease (CKD) underwent FE-MRA and non-contrast CT for pre-procedural vascular assessment. The concordance correlation coefficients (CCCs) for image registration between the original FE-MRA and fused image in each dimension were 0.9997 (ρ=0.9997, 95% CI 0.9996-0.9998), 0.9993 (ρ=0.9994, 95% CI 0.9992-0.9995) and 1 (ρ=1, 95% CI 0.9999-1.0000) in X, Y and Z dimensions respectively. Interobserver correlation of image registration was excellent (all ICCs >0.99). No ferumoxytol-related adverse events occurred in any patient.DISCUSSION
The fusion of FE-MRA and non-contrast CT offers highly accurate and reproducible image registration for pre-procedural vascular planning. The use of ferumoxytol with non-contrast CT is especially advantageous in patients with renal impairment where iodinated contrast media and gadolinium based contrast agents are contraindicated and where vascular calcification may be florid. As the purpose of our study was to report the feasibility of fusion images, the patient sample size is relatively modest. Further, because the method for validation of the fusion offsets between vasculature and calcifications has not been previously established, our work was limited to evaluations of the offsets of pacemakers and catheters between original and fused images. Although the registration takes less than 5 minutes, the image segmentation is more time consuming and will require workflow optimization strategies for time sensitive clinical applications. A deep learning strategy may be relevant for future implementation of image fusion techniques.CONCLUSIONS
Based on our initial experience, accurate 3D fusion can be generated by combining FE-MRA and non-contrast CT. This approach holds promise to combine the complementary strengths of MRI and CT, while avoiding their respective risks and limitations.Acknowledgements
No acknowledgement found.References
1. Rogers T, Waksman R. Role of CMR in TAVR. JACC Cardiovasc Imaging 2016;9(5):593-602.
2. Nguyen KL, Moriarty JM, Plotnik AN, et al. Ferumoxytol-enhanced MR Angiography for Vascular Access Mapping before Transcatheter Aortic Valve Replacement in Patients with Renal Impairment: A Step Toward Patient-specific Care. Radiology 2017:162899.
3. Toth GB, Varallyay CG, Horvath A, et al. Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney Int 2017;92(1):47-66.
Figures