3335
Cardiac MRI detects functional deterioration prior to apoptosis in a mouse model of doxorubicin-induced cardiotoxicity using tissue phase mapping1Radiology, Northwestern University, Chicago, IL, United States
Synopsis
Doxorubicin-induced cardiotoxicity is an important limiting factor preventing dose-effective cancer treatment, and the associated cardiac sequelae appears to be mediated through cardiac myocyte apoptosis. Multiple studies have suggested there is potential for cardiovascular MRI (CMR) multiparametric analysis to detect doxorubicin-induced cardiotoxicity. In the current study, we performed multiparametric CMR including DENSE strain assessment and tissue phase mapping (TPM) for myocardial velocity measurement in a mouse model of doxorubicin-induced cardiotoxicity. Our results suggest that advanced CMR functional assessment shows promise in identifying treatment-related decrease in myocardial longitudinal systolic and diastolic velocity prior to the onset of cardiac myocyte apoptosis.
Introduction
Doxorubicin-induced cardiotoxicity is an important limiting factor preventing dose-effective cancer treatment, and the associated cardiac sequelae appears to be mediated through cardiac myocyte apoptosis.(1) Both pre-clinical and clinical studies have suggested there is potential for cardiovascular MRI (CMR) multiparametric analysis to detect doxorubicin-induced cardiotoxicity, specifically through T1 and T2 myocardial tissue characterization.(2) Studies using echocardiography and CMR have also suggested circumferential and global longitudinal strain are reduced following treatment with doxorubicin.(3) In the current study, we have expanded a standard multiparametric CMR cardiotoxicity examination to now include DENSE strain assessment and tissue phase mapping (TPM) for myocardial velocity measurement. In this pilot study, we have applied this protocol in a mouse model of doxorubicin cardiotoxicity and hypothesize that myocardial functional characterization will allow for the identification of doxorubicin-induced cardiotoxicity prior to cardiac myocyte apoptosis.Methods
Four 16 week-old female C57BL/6 mice were included in the study. Each mouse was treated with a total of 25 mg/kg of doxorubicin over 5 weeks via subcutaneous implantations of slow-release doxorubicin pellets (daily dose ranges between 0.4 and 0.8 mg/kg/day). The mice were imaged prior to treatment and then at week five of treatment at a cumulative dose of 25 mg/kg. All MRI was performed on the 7T Clinscan system (Bruker,Germany).
Mice were maintained at 1.25% isoflurane during MRI. Images were acquired using a 3 channel surface coil and a MR-compatible physiological monitoring and gating system (SA instruments, Inc., Stony Brook, NY). The MRI protocol included cine MRI, cine DENSE MRI, cine TPM, pre-contrast and post-contrast T1 mapping. Cine MRI was performed using multi-slice SSFP MRI covering the entire LV while the rest of the imaging was performed in a single mid-ventricular short-axis slice. The images were exported to a workstation and analyzed using Segment software (Medviso, AB) to calculate ejection fraction(EF). Peak circumferential (Ecc) and radial (Err) strain were calculated from DENSE images using dedicated software built in MATLAB (Mathworks, Inc.). (4) TPM was performed using 2D cine black-blood segmented phase contrast MRI with prospective ECG and respiratory triggering (TE/TR =3.4/5.2 ms, image resolution =117x117x1000 μm3, flip angle=150, averages=3, in-plane and through-plane VENC= 4 cm/s and through-plane VENC= 4cm/s). TPM analysis was performed in MATLAB. (5) T1 mapping was performed using a compressed-sensing (CS) accelerated cardio-respiratory triggered spiral Look-Locker pulse sequence with Block LOw-rank Sparsity with Motion-guidance (BLOSM) reconstruction. Imaging parameters included: acceleration rate = 2, time between inversions = 7 s, slice thickness = 1 mm, number of spiral interleaves for full Nyquist sampling = 84, interleaves per heartbeat = 3, resolution = 100x100x1000 μm3. The undersampled images were reconstructed using Block LOw-rank Sparsity with Motion-guidance (BLOSM). Gadolinium (0.2 mmol/kg) was administered by three intraperitoneal injections and T1 mapping was performed pre-contrast and 5 min post-injection of gadolinium. Image analysis was performed using MATLAB. Myocardial ECV was obtained by multiplying partition coefficient with (1-Hematocrit).
Following imaging, mice were sacrificed and hearts were harvested for histopathologic assessment. The evaluation included qualitative review of sections utilizing terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL) to detect apoptotic cells.
Results
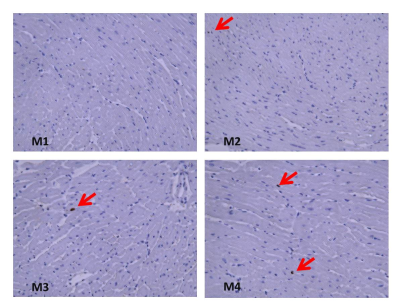
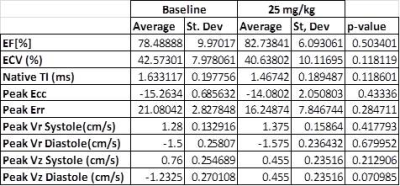
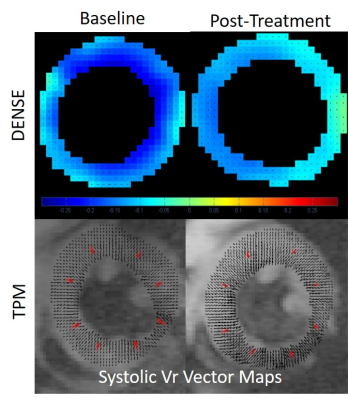
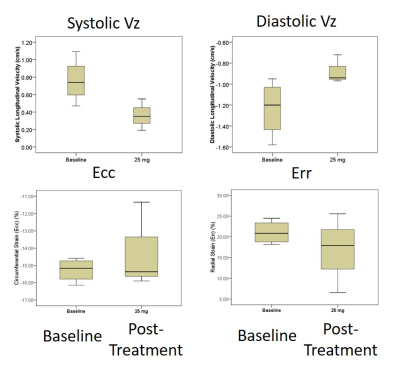
Histopathology results demonstrate minimal apoptosis in all mice (~1 apoptotic cell/high power field), suggesting early-stage cardiotoxicity (Figure 1). There was no statistically significant difference in any examined CMR parameters between baseline and post-treatment imaging (Table), although our power to detect differences is low in this small cohort. There was a clear qualitative trend in reduced magnitude post-treatment longitudinal diastolic and systolic velocities suggesting this may be an early marker of treatment effects (Figure 2). Radial and circumferential strain measured by DENSE demonstrated increased standard deviation after treatment, suggesting these parameters may also be impacted by treatment effects.Conclusions
In a mouse model of doxorubicin-induced cardiotoxicity, advanced CMR functional assessment shows promise in identifying treatment-related decrease in myocardial longitudinal systolic and diastolic velocity prior to the onset of cardiac myocyte apoptosis. Of note, these parameters were assessed at a single slice in the left ventricle which also suggests relatively high sensitivity of this technique. Work is ongoing to increase the cohort size to better power our statistical analysis to detect differences in these parameters. Additional histologic and electron microscopy evaluation will be performed to help identify inflammatory and subcellular changes indicative of cardiotoxicity with the goal of optimizing our imaging approach and timing during the course of treatment.Acknowledgements
References
1. Zhao L, Zhang B. Doxorubicin induces cardiotoxicity through upregulation of death receptors mediated apoptosis in cardiomyocytes. Scientific reports 2017;7:44735.
2. Farhad H, Staziaki PV, Addison D, et al. Characterization of the Changes in Cardiac Structure and Function in Mice Treated With Anthracyclines Using Serial Cardiac Magnetic Resonance Imaging. Circ Cardiovasc Imaging 2016;9(12).
3. Thavendiranathan P, Wintersperger BJ, Flamm SD, Marwick TH. Cardiac MRI in the Assessment of Cardiac Injury and Toxicity From Cancer Chemotherapy. A Systematic Review 2013;6(6):1080-1091.
4. Gilliam AD and Epstein FH. Automated motion estimation for 2-D cine DENSE MRI. IEEE transactions on medical imaging. 2012;31:1669-81.
5. Jung B., Markl M., Föll D., Hennig J.; Investigating myocardial motion by MRI using tissue phase mapping, European Journal of Cardio-Thoracic Surgery, Volume 29, Issue Supplement_1, 1 April 2006, Pages S150–S157, DOI: https://doi.org/10.1016/j.ejcts.2006.02.066
Figures