3334
Systolic and Diastolic Strain Abnormalities are Observed in Spontaneously Diabetic Non-Human Primates with Diastolic Dysfunction and Preserved Ejection Fraction.1Translational Biomarkers, MRL, MSD, Singapore, Singapore, 2Sichuan Primed Shines Bio-tech Co. Ltd., Chengdu, China, 3Department of Radiology, West China Hospital, Sichuan University, Chengdu, China, 4Department of Radiology, Washington University School of Medicine, St Louis, MO, United States, 5Translational Biomarkers, MRL, Merck & Co., Inc., West Point, PA, United States
Synopsis
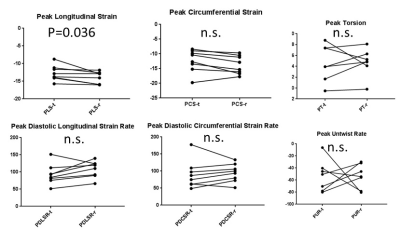
Non-human primate (NHP) models of cardiovascular disease or metabolic disorder offer a unique framework to evaluate novel therapeutics. Herein, we perform cardiac strain MRI on a 3T MRI scanner to characterize systolic and diastolic function in naïve and spontaneously diabetic NHPs with diastolic dysfunction (T2DM-DD) and preserved ejection fraction (which may represent an early phenotype of HFpEF). In addition, the naïve animals were imaged twice to perform test-retest analysis of the strain-based biomarkers. Peak strains and peak diastolic strain-rates (both circumferential and longitudinal) are significantly impaired in the T2DM-DD monkeys compared to the naïve monkeys indicating impaired systolic and passive diastolic function. In addition, peak untwist rate is also decreased depicting impairment in active diastolic function as well. The test-retest results in the naïve animals show that all biomarkers, with the exception of peak longitudinal strain, are reproducible.
Authorship
Smita Sampath, Li Gong, and Yushu Chen have equally contributed to this work.Introduction
Heart failure with preserved ejection fraction (HFpEF) is a growing epidemic that represents a great burden to society. With growing comorbidities such as diabetes, obesity and aging, studies predict that over 65% of patients hospitalized for heart failure in 2020 will suffer from HFpEF [1]. Currently, there are no effective treatments in the clinic for HFpEF, creating a great need to develop novel therapeutics that demonstrate efficacy in this patient population. Pre-clinical animal models that closely mimic human HFpEF will potentially play a significant role in the understanding of the pathogenesis and in pre-clinical investigations of novel experimental compounds. Metabolically driven non-human primate (NHP) models of cardiac disease that recapitulate the human HFpEF phenotype at an early stage of the disease may be pivotal to understand the mechanistic pathways involved in longitudinal disease progression and mechanism of action of experimental compounds. Quantitative characterization of these disease models using clinically relevant biomarkers will be critical in the evaluation of the model itself, as well as in characterizing disease progression and the assessment of response to treatment. Herein, we perform cardiac MRI in naïve and spontaneously diabetic NHPs with diastolic dysfunction (T2DM-DD) and preserved ejection fraction (which may represent an early phenotype of HFpEF), where strain-based diastolic and systolic biomarkers are used to further characterize the two NHP cohorts.Methods
Animals and Equipment: 9 healthy rhesus monkeys (weight: 9.5±1 kg (mean±SD), age: 11±1 years) and 9 T2DM-DD (weight: 11±1 kg, age: 14±2 years) rhesus monkeys with diabetes (based on IVGTT) and diastolic dysfunction (based on echo parameter cutoffs : E/A>1, E'/A'<1.1,E/E'>11, E'<7cm/s with preserved ejection fraction (EF≥65%)) were imaged using the 3-T Trio MR imaging scanner (Siemens Medical Solutions, Germany) housed at West China hospital. All animals had normal lipid profile, normal hepatic and renal function and were normotensive. Images were obtained using a standard cardiac phased array coil. Cardiac Function MRI: A gated, multi-phase, segmented gradient echo pulse sequence with a 1-2-1 SPAMM tagging preparation sequence [2] was employed to image myocardial displacement. Two sets of tagging datasets with orthogonal in-plane tagging modulations were acquired for three SA slices and for the 2-chamber and 4-chamber LA slices. The selected imaging parameters were: FOV: 160 mm X 160 mm, imaging matrix: 192 X 192, slice thickness: 4 mm, segments=4, echo spacing: 6.6 ms, bandwidth: ±511Hz/pixel, TR/TE: 26.36 ms/3.12 ms, flip angle: 10°, tag separation: 4 mm, phases: 16. Experimental Design Imaging was performed once on the 9 T2DM-DD monkeys, and twice on the 9 healthy monkeys for test-retest analysis of the biomarkers. The two repeated imaging sessions were conducted on different days. Data Analysis: All data analyses were conducted using in-house MATLAB HARP [3] analysis tools. Three systolic biomarkers (peak circumferential and longitudinal strain, and torsion) and three diastolic biomarkers (peak circumferential and longitudinal diastolic strain rate and untwist rate) were quantified to characterize cardiac function in these animals.Results and Discussion
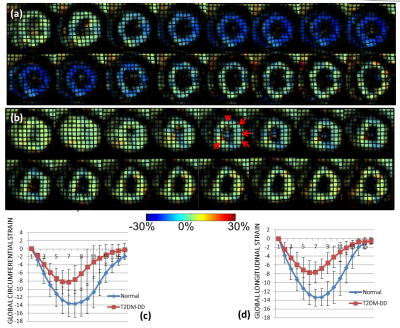
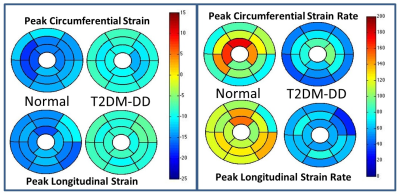
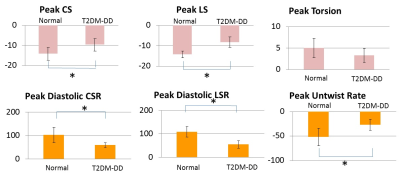
Peak strains (both circumferential and longitudinal) are significantly impaired in the T2DM-DD monkeys compared to the naïve monkeys despite preserved ejection fraction (see Figs. 1c and d, and Fig. 3). This decrease in strain is homogenous across all regions in the left ventricle (see Bulls eye plots in Fig 2 and representative data in Fig.1 a and b), indicating that systolic function is also affected in the early stages of HFpEF, likely due to stiffening[4]. This notion is further corroborated by the observed significant and homogenous reduction in peak diastolic strain rates observed in the T2DM-DD animals (see Bulls eye plots in Fig. 2 and Fig. 3) indicating increased passive stiffness in the animals. In addition, peak untwist rate is also decreased depicting impairment in active diastolic function as well. The test-retest results in the naïve animals show that all biomarkers, with the exception of peak longitudinal strain, are reproducible (see Fig. 4)Conclusion
We have successfully characterized strain-based biomarkers in a spontaneous NHP model of metabolically driven diastolic dysfunction with preserved ejection fraction. The results show both systolic and diastolic impairment in the diseased animals indicative of myocardial stiffening. The strain-based functional biomarkers may offer translatable non-invasive early markers of cardiac remodeling for preclinical investigation of therapeutic responses.Acknowledgements
No acknowledgement found.References
[1] Oktay AA et. al. Current Heart Failure Reports 2013 [2] Axel L et. al. Radiology 1989 [3] Osman et. al. IEEE TMI 2000 [4] Kraigher-Krainer E et. al. JACC 2014Figures