3333
MR-compatible Echocardiography and Predictive Slice Tracking Towards Ultrasound-driven Cardiovascular MRI1Division of Radiology, Geneva University Hospitals, Geneva, Switzerland, 2Radiology, Universitätsspital Basel, Basel, Switzerland
Synopsis
MR imaging of cardiac valves is challenging as out-of-plane motion limits the use of 2D cine acquisitions. Ultrasound is well accepted as the clinical tool for observing valve motion. Here we suggest a hybrid imaging technology using in-bore ultrasound for direct observation of the motion of interest and subsequent on-the-fly adaptation of the MR slice position. MR-compatibility and workflow of echocardiography in-situ was demonstrated on a volunteer. Dynamic correction of motion in MR images was quantified with a moving phantom. Future-predicting algorithms yielded a reduction of apparent motion amplitude by a factor of twenty and dramatically improved “cine” image sharpness versus uncorrected data.
Background
Cardiac MRI has the potential to depict the cardiac valves with high resolution and gives functional information. However, morphology that is key for planning surgical or minimally invasive treatment, is assessed by echocardiography. Quality of ultrasound images is patient dependent and the geometry is often limited to a single plane. Therefore, improving the accuracy of MRI to assess valve morphology would significantly advance valvular disease evaluation. Navigated/self-navigated sequences are under development. However, valve MRI with ultrasonography-equivalent quality remains difficult. We suggest an ultrasound-guidance method for motion compensation during MR acquisition with in-bore ultrasound (US), and a future-predicting algorithm illustrated in high-resolution cine MRI of a ‘breathing’ phantom.Methods
Ultrasound used a clinical Siemens ACUSON Antares. A P.7.3-like linear-array probe (192-element) was built MR-compatible with advanced electromagnetic shielding and the cable, grounded with the Faraday cage, transferred signals from the in-situ probe. US images were sent in near real-time to an external PC, which extracted the average motion vector in a user-defined ROI using optical-flow-based tracking (OpenCV2.3.1), from the second-harmonic US image with tracking via acoustic speckle shift. This was communicated to the MR system on-the-fly to adjust the slice position during acquisition.
A non-parametric algorithm predicted the future position of the moving object (+60ms) via fitting of previous datapoints. A second-degree polynomial was fitted to the last n tracked coordinates versus time, and used to predict future coordinates. In order to follow respiratory movement, it was determined empirically that the best polynomial anticipation is a 6-point interpolation by a 2nd degree polynomial.
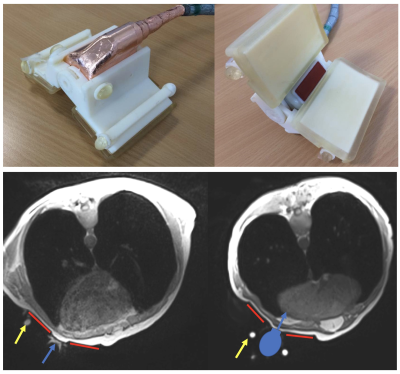
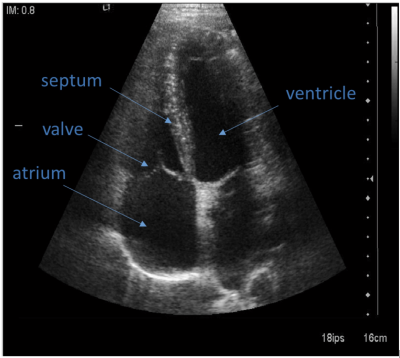
In-vivo imaging (healthy volunteer, prone position, spine and Body18 coil combined) used a 3D VIBE breathold to localise the imaging plane of the US probe (Fig1). The probe was held in place in a 3D-printed support complete with 6 MRI-visible markers for localisation of the US plane on the MRI image and probe positioning with 3 degrees of freedom. A customised wedge platform was placed on the MRI table to allow optimal prone position of the patient for the best acoustic path. Cardiac valve US was acquired during MRI acquisition.
Quantification of MR “cine” image improvement, however, required a gold-standard static image which is only achievable in a phantom. A gel-filled bottle was placed centrally above a 11cm loop coil in a water bath positioned inside a Siemens 3T PRISMA scanner. The bottle was attached to an Innomotion robot simulating respiratory motion (amplitude 20mm, head-foot, Fig2). Spatial contrast was produced by air bubbles included in the gel, size range 1-5mm. A conventional cardiac-triggered segmented balanced-SSFP sequence was modified to adapt slice positioning in quasi-real-time according to information received from the ultrasound tracking. Real-time bSSFP images were acquired to determine the optimum fitting. High-resolution phantom ‘cine’ bSSFP was acquired with acquisition longer than breath-hold (1min). The cine images were assessed for visual sharpness and quantified with a Canny filter edge pixels and by image subtraction.
Results
In vivo, there were no confounding US-based artifacts on MRI nor on the US during MR acquisition. Cardiac valves were visible in-vivo and trackable on ultrasound images during MR acquisition (Fig3). The acoustic window did not interfere with the ECG patches.
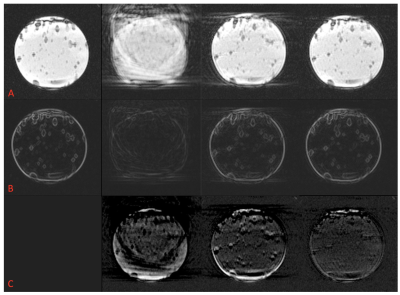
In the phantom, Fig4 shows the time dimension of 200 real-time images. Quantitatively, the standard deviation of motion was 8.86mm uncorrected, 0.68mm on-the-fly, 0.53mm predictive. Visually, cine image quality was significantly improved with predictive correction (fig5A) and the edge sharpness increase is clear on gradient images (fig5B). Visible motion artifacts are seen at the superior/inferior edges of the phantom with the on-the-fly correction and were reduced with predictive correction throughout the cardiac cycle.
Canny filter quantification: static 437, uncorrected 194, on-the-fly correction 393, predictive 465 pixels. Mean±standard deviation signal intensity difference from static: uncorrected -36±233, on-the-fly -6±200, predictive 2±97 (fig5C). The predictive correction image was almost identical to the static gold-standard.
Discussion and Conclusions
This protocol corrects, at present, the principal axis of motion. Application in vivo will require fat saturation and registration of the US imaging plane to the MR frame. However, the method corrects all movements combined, no matter what source (respiration, contraction and valve function) as the anatomy of interest is directly observed by the US and there is no rejection of data.
With this multimodality method, we corrected respiratory phantom motion-blurring artifacts in long acquisition time, high-resolution cine acquisitions. On-the-fly correction was improved by the addition of a prediction algorithm using polynomial fitting. Workflow of MR-compatible, in-bore US valve imaging of diagnostic quality was demonstrated and the correction from the tracking algorithm validated in the phantom by observing directly the motion of interest.
Acknowledgements
No acknowledgement found.References
No reference found.Figures