3328
MRI-derived myocardial strain in patients with mild cognitive impairment (MCI)1Radiology, Yuhuangding Hospital, Qingdao University School of Medicine, Yantai, China, 2Radiology, Northwestern University, Chicago, IL, United States
Synopsis
Our data show that patients with mild cognitive impairment (MCI) have a lower regional peak myocardial strain and peak systolic strain rate at left ventricle (LV) as compared with healthy controls. Patients with MCI seem to have a heavier burden of subclinical cardiovascular diseases (CVDs).
Background
Mild cognitive impairment (MCI) and dementia, are prevalent in older adults (1). Alzheimer's disease (AD) is the leading reason of MCI and dementia and also serves as the sixth-leading cause of death in the United States. Cardiovascular disease (CVD) and its risk factors, which are also age-related conditions, have been found to be related with the development of MCI/dementia/AD (2). Unfortunately, cardiovascular indicators presenting the global cardiac function, seem to be insufficient to connect subclinical CVD to the development of MCI/dementia/AD in current publications. It is well known that myocardial dysfunction could be regional in the subclinical stage of CVD resulting in a minimally apparent decrease in global cardiac function indices, such as left ventricle (LV) ejection fraction (EF). Therefore, we hypothesize that patients with MCI have characteristic MRI-derived regional myocardial deformation indices as compared with healthy controls.Methods
With approval of local ethics review committee, 7 patients (3 female, 63+/- 7 years) with MCI and 10 healthy controls (4 female, 62 +/- 8 years) were recruited and consented for a non-contrast cardiac MRI scan using a 3T scanner (Signa Excite, GE healthcare). All participants did not have documented structural heart diseases. MCI was diagnosed by a neurologist based on criteria published by Alzheimer's Association (www.alz.org). Cine images were acquired by using a Fast Imaging Employing Steady-state Acquisition (FIESTA) sequence at two-chamber, three-chamber and short-axis views. The short-axis cine images covered the heart from base to apex with 10-12 slices. Imaging parameter included: TR/TE = 3.6/1.6 msec, FOV = 380x380 mm, Matrix = 512x512, Slice thickness = 8 mm. Twenty retrospective phases were constructed within a cardiac cycle.
All images were analyzed using Circle CVI42 (Calgary, Canada). MRI-derived global and regional cardiac function and motion indices, such as LVEF, strain, strain rate, time to peak at radial and circumferential directions were calculated. Regional motion indices were mapped on an AHA 16-segment LV model. Those indices were compared between two participant groups using t-tests. Intra- and inter-observer variations for strain measurements were also evaluated. Statistical analysis was performed by using SPSS software (Version 22.0). A p value < 0.05 was considered as having statistical significance.
Results
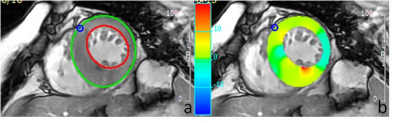
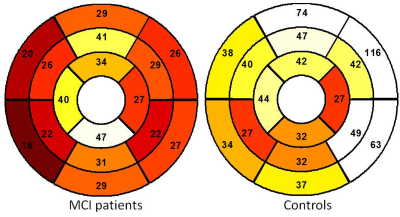
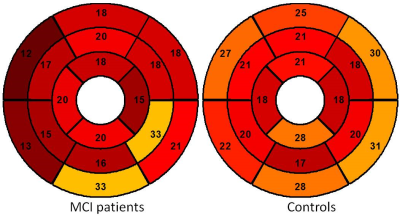
All 17 scans were completed. Figure 1 shows typical images of contours and strain fields. Compared with asymptomatic controls, patients with MCI have lower radial (29% +/-24% vs. 46% +/- 43%, p < 0.001) and circumferential (19% +/-17% vs. 22% +/-14%, p = 0.047) strain. MCI patients also have lower peak systolic radial strain rate at radial (2.59 +/- 2.18 s-1 vs. 3.87+/- 3.36 s-1, p < 0.001) and circumferential (2.77+/- 2.26 s-1 vs. 5.97+/- 3.99 s-1, p <0.001) directions. Figure 2 and 3 show regional distributions of peak strain for two subject groups. There were no significant differences of LVEF between two subject groups (52% +/-13% vs. 54%+/-15%, p = 0.56). Good intra- and inter-observer agreement on measuring regional myocardial motion indices was also observed.Discussion
In the present study, we found that patients with MCI have lower regional peak strain and lower peak systolic radial strain rate than asymptomatic controls. Such a characteristic regional motion pattern suggests that MCI patients may have a heavier subclinical CVD burden than healthy controls.
Recently, quantitative myocardial motion indices, such as displacement, velocity, strain and strain rate, have been adopted to more comprehensively describe regional myocardial motion abnormalities in patients with clinical or subclinical CVDs (3, 4). CVDs and neurodegeneration are two major threatens to older populations. However, existing studies shows unstable relations between global cardiac indices and MCI/Dementia/AD. In Framingham study, cardiac index (CI) was found to be linearly related to the incidence of MCI/dementia/AD while LVEF did not demonstrate such a relationship (5, 6). To the best our knowledge, it is the first study to relate regional myocardial deformation to MCI.
Our study has limitations. First, due to long scan time, cardiac MRI could not be performed for many dementia/AD patients. As a result, the subject group was composed of MCI patients. Second, the sample size is small. We only recruited 7 patients. However, a further study with a larger sample size aiming at investigating the relations between subclinical CVDs and cognitive impairment is warranted. Third, we only balance most important factors, such as age, for subject groups. More traditional cardiovascular risk factors will be enrolled for data analysis in large scale studies. Fourth, we will improve our protocol to include long-axis strain analysis in the future.
Conclusion
Compared to healthy controls, patients with MCI have lower MRI-derived regional myocardial strain and strain rate.Acknowledgements
No acknowledgement found.References
1. Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1-2):125-32.
2. Schievink SHJ, van Boxtel MPJ, Deckers K, van Oostenbrugge RJ, Verhey FRJ, Kohler S. Cognitive changes in prevalent and incident cardiovascular disease: a 12-year follow-up in the Maastricht Aging Study (MAAS). European heart journal. 2017.
3. Pandey A, Park B, Martens S, et al. Relationship of Cardiorespiratory Fitness and Adiposity With Left Ventricular Strain in Middle-Age Adults (from the Dallas Heart Study). Am J Cardiol. 2017;120(8):1405-9.
4. Luetkens JA, Doerner J, Schwarze-Zander C, et al. Cardiac Magnetic Resonance Reveals Signs of Subclinical Myocardial Inflammation in Asymptomatic HIV-Infected Patients. Circ Cardiovasc Imaging. 2016;9(3):e004091.
5. Jefferson AL, Beiser AS, Himali JJ, et al. Low cardiac index is associated with incident dementia and Alzheimer disease: the Framingham Heart Study. Circulation. 2015;131(15):1333-9.
6. Jefferson AL, Himali JJ, Au R, et al. Relation of left ventricular ejection fraction to cognitive aging (from the Framingham Heart Study). The American journal of cardiology. 2011;108(9):1346-51.
Figures