3325
Comparison of Dual- and Tri-band Excitation for MS-CAIPIRINHA-accelerated First-Pass Myocardial Perfusion ImagingTobias Wech1,2, Tina Urbanek1,3, Andreas Max Weng1, Daniel Stäb4, Peter Speier5, Thorsten Alexander Bley1, and Herbert Köstler1,2
1Department of Diagnostic and Interventional Radiology, University Hospital Würzburg, Würzburg, Germany, 2Comprehensive Heart Failure Centre, University Hospital Würzburg, Würzburg, Germany, 3Fakultät Ingenieurwissenschaften, Hochschule für Technik und Wirtschaft des Saarlandes, Saarbrücken, Germany, 4The Centre for Advanced Imaging, The University of Queensland, Brisbane, Germany, 5Magnetic Resonance, Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
MS-CAIPIRINHA is a valuable technique to extend the anatomical coverage in myocardial first-pass perfusion imaging. In our previous studies, dual-band excitation was applied in conjunction with three SR-prepared acquisition blocks per RR interval to obtain six 2D-slices with a temporal resolution of one heartbeat. Especially in obese patients, however, the SNR occasionally turned out to be marginal, ultimately complicating the assessment of perfusion defects. In this work, an according approach using tri-band RF excitation was tested with respect to potential SNR benefits.
Target audience
Clinicians interested in cardiac perfusion and
MR physicists interested in using MS-CAIPIRINHA.Purpose
MS-CAIPIRINHA is a valuable means to extend the anatomical coverage in first-pass myocardial perfusion imaging [1, 2, 3]. In earlier studies [4, 5], three saturation-recovery (SR) preparations were used per RR-interval, each followed by an undersampled dual-slice MS-CAIPIRINHA acquisition. The parallel imaging (PI) reconstruction then yielded six 2D-slices with a temporal resolution of one RR-interval. Especially in obese patients, however, the SNR occasionally turned out to be marginal for a robust diagnosis of perfusion defects.In this work, the technique was compared to an alternative approach: Two SR preparations were performed per RR-interval and each was followed by an undersampled MS-CAIPIRINHA with tri-band excitation. Aim of the study was to evaluate whether the new approach results in a higher signal-to-noise-ratio (SNR).
Methods
The TurboFLASH-based MS-CAIPIRINHA prototype (see above, [4]) utilized dual-band RF pulses to excite two slices at the same time. The simultaneously excited slices were shifted with respect to each other by ½ FOV by toggling the RF phase in the second slice between 0 and π.In this work, tri-band RF pulses were implemented in order to excite three slices at the same time. The RF phases in the three slices were varied according to $$$\phi_1(j)=0$$$, $$$\phi_2(j)=\frac{2\pi}{3}j$$$ and $$$\phi_2(j)=\frac{4\pi}{3}j$$$ with the excitation index $$$j$$$, in order to shift the slices by ⅓ FOV with respect to each other and equally distribute the slice aliasing along the phase encoding direction. Two SR-preparations were applied per RR-interval to obtain six slices, in accordance with the dual-band approach.
First, both techniques were compared in a first-pass perfusion investigation of a patient with primary hyperaldosteronism. Data were acquired on a 3T whole-body MR-scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) equipped with a body and spine phase array coil using 34 elements in total. For each measurement, a 10 ml bolus of gadoterate meglumine (Dotarem, Guerbet, Aulnay-sous-Bois, France) was injected and observed over 60 RR-intervals. The overall PI acceleration factor corresponded to 5 (RCAIPI = 2, RInPlane = 2.5) for dual-band and to 4 (RCAIPI = 3, RInPlane = 1.3) for tri-band excitation. TGRAPPA [6] was used for offline image reconstruction in MATLAB (MathWorks, Natick, MA, USA). The remaining imaging parameters included: Spatial resolution = 3.1 mm x 2.5 mm, TE=1.06, TR = 2.33, FA = 10°, slice thickness = 8mm, phase encoding steps (PES) / RR-interval = 135 for the dual-band and: Spatial resolution = 3.1 mm x 2.5 mm, TE=1.38, TR = 2.65, FA = 10°, slice thickness = 8mm, PES / RR-interval = 168 for the tri-band acquisition.
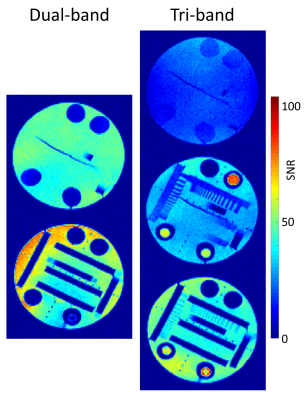
An SNR study was then performed for a quantitative comparison of the dual- and the tri-band techniques. Phantom data were acquired on the same scanner. For an objective comparison, the total number of PES was kept equal for both acquisitions. The dual-band MS-CAIPIRINHA measurement was performed using three SR preparations, each followed by 45 phase-encoding steps (135 PES in total, Rtotal=5), while the tri-band acquisition featured two SR-preparations, each followed by 68 phase-encoding steps (136 PES in total, Rtotal=4). A measurement of the noise amplitude and correlation in the 34-channel phased-array receiver coil was performed for both approaches and the method described in [7] was used to obtain SNR maps. Remaining imaging parameters were adjusted as follows: spatial resolution = 2.3 mm x 1.9 mm; TE = 1.49; TR = 2.79; flip angle =10°.
Results
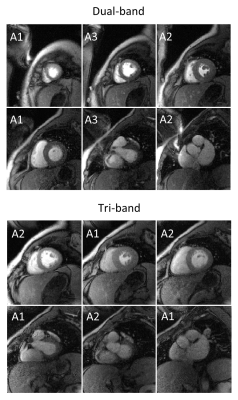
The results of the first-pass perfusion acquisitions are depicted in Fig. 1. Both techniques resulted in an overall good image quality. No considerable differences in artifact level and image sharpness are apparent throughout all frames of the image series. The maps in Fig. 2 reveal an overall higher SNR for the dual-band approach. The average SNR across all pixels of all slices was 27±13 for the tri-band and 41 ± 16 for the dual-band pulse.Discussion & Conclusion
The
simulations performed in our study compared dual- and tri-band MS-CAIPIRINHA
with respect to their usability in first-pass myocardial perfusion imaging.
Both techniques yielded six slices with a temporal resolution of one
RR-interval, however, the SNR was significantly lower for the tri-band
excitation. This is most likely due to the comparably small slice distances in
cardiac MRI, which result in a limited variation of the coil sensitivities and
ultimately in an elevation of the g-factor. The dual-band acquisition with three SR preparations is therefore the method of choice. This is also justified by a shorter acquisition window for each multi-slice time frame, which comes with less cardiac motion and less signal variation due to the ongoing recovery of the magnetization.