3324
Low-Rank plus Sparse Matrix Decomposition for Accelerated Radial MS-CAIPIRINHA in First-Pass Myocardial Perfusion Imaging1Department of Diagnostic and Interventional Radiology, University Hospital Würzburg, Würzburg, Germany, 2Comprehensive Heart Failure Centre, University Hospital Würzburg, Würzburg, Germany, 3Department of Internal Medicine I, University Hospital Würzburg, Würzburg, Germany, 4The Centre for Advanced Imaging, The University of Queensland, Brisbane, Germany, 5Magnetic Resonance, Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
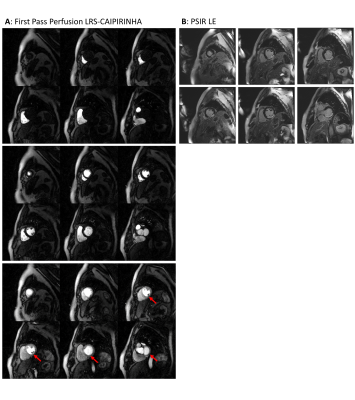
The anatomical coverage in first-pass myocardial perfusion imaging was extended by applying undersampled radial MS-CAIPIRINHA and a model-based reconstruction exploiting low-rank plus sparse matrix decomposition. The technique was tested in a patient with acute left ventricular myocardial infarction, yielding six short-axis slices from base to apex with a temporal resolution of one heartbeat. The reconstructed images exhibited a quality which is comparable to the conventional approach of acquiring only three slices per RR interval.
Target audience
Clinicians interested in cardiac perfusion and
MR physicists interested in using MS-CAIPIRINHA.Purpose
The anatomical coverage in first-pass myocardial perfusion imaging can effectively be extended by applying the simultaneous multi-slice Parallel Imaging (PI) technique MS-CAIPIRINHA [1, 2]. For realistic acquisition times, in-plane PI acceleration [2] and/or non-Cartesian trajectories [3, 4] are typically used in addition. Even on 3T scanners, however, the signal-to-noise ratio (SNR) can be marginal, especially when applying the named techniques in obese patients. In this work, we developed an image reconstruction technique for accelerated radial MS-CAIPIRINHA acquisitions based on low-rank plus sparse matrix decomposition and applied it to first-pass myocardial perfusion imaging.Methods
The applied ECG-gated TurboFLASH prototype [5]
utilized a dual-band pulse to excite two slices simultaneously, while the RF phase
of the first slice was kept unmodulated and the phase of the second slice was
toggled between 0° and 180°. Radial readouts were implemented with a golden-angle
increment and no resets of the projection angle for each RR-interval. Three
saturation recovery (SR)-prepared acquisition blocks (one block per
dual-slice/slice-pair) consisting of 52 consecutive projections were acquired
per RR-interval (~60 RR-intervals in total).
The hereafter-proposed algorithm
was then used to obtain six images per RR-interval out of this data: To
reconstruct the highly undersampled radial MS-CAIPIRINHA dataset, a low-rank
plus sparse matrix decomposition of the temporal image series [4] was used (referred to as "LRS-CAIPIRINHA"):
$$ \min_{L_{sl},S_{sl}} \; \biggl\Vert \biggl(\sum_{sl=1}^2
\Phi_{sl} \, E_{sl} \, (L_{sl}+S_{sl}) \biggr)-y \, \biggr\Vert_2^2 +
\alpha \sum_{sl=1}^2 \Vert L_{sl} \Vert_* + \beta \sum_{sl=1}^2 \Vert
\mathcal{F} S_{sl} \Vert_1 $$
$$$y$$$ represents the measured k-t-space multi-coil data for one of the three dual-slice acquisitions. $$$L_{sl}$$$ and $$$S_{sl}$$$ correspond to the low-rank and the sparse component of the temporal image series for one of the two slices $$$sl$$$, in accordance to the single-slice definition in [6]. $$$E_{sl}$$$ is the encoding operator, which incorporates an inverse Fourier transform, the re-gridding back to the initial radial projections and the superposition of the coil sensitivities to obtain multi-coil data. The coil sensitivities for each slice were determined using an oversampled temporal average, i.e. data were pooled from all RR-intervals and gridded into one single k-space. $$$\Phi_{sl}$$$ performs the applied CAIPIRINHA phase modulation, $$$\Vert \; \Vert_*$$$ represents the nuclear norm and $$$\alpha, \beta$$$ balance the weighting of the different constraints. The latter parameters were chosen based on visual inspection of the reconstructed images. $$$\mathcal{F}$$$ performs a 1D Fourier transform in the temporal domain to additionally sparsify $$$S_{sl}$$$. Projection onto convex sets (POCS) was used to perform the optimization. 50 iterations were applied, and the reconstruction of the whole dataset took approximately five hours on an Intel Core i7-3820 CPU @ 3.60 GHz without parallel computation.
The imaging technique was tested on a 3T MR scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) in a patient with known acute left ventricular myocardial infarction (BMI = 26.6). The study was approved by our local ethics committee, and the patient gave written informed consent prior to scanning. Imaging parameters were chosen as follows: Slice distance between adjacent slices =12.3mm, slice distance between simultaneously acquired slices = 37.0mm, saturation preparation & ECG triggering, TE = 1.5ms, TR = 2.6ms, slice thickness = 8mm, in-plane resolution = 1.9mm x 1.9mm, 52 radial projections per image pair, image matrix = 160 x 160. A compact bolus of gadoterate meglumine (Dotarem, Guerbet, Aulnay-sous-Bois, France) was injected, and imaging was performed over 60 RR-intervals with 3x2 short-axis slices, acquired within each interval. The measurement was started with an initial breath-hold of the patient, which lasted for approximately 20-25 of the 60 heartbeats of the entire investigation. To provide a reference for the size and the shape of the myocardial scar, the reconstructed perfusion series were analyzed in conjunction with the phase-sensitive inversion recovery late-enhancement (PSIR-LE) images acquired during the clinical MR investigation.