3318
Tissue Phase Mapping for Assessment of Biventricular Myocardial Motion in Pediatric Patients with Repaired Tetralogy of Fallot1Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, IL, United States, 2Feinberg School of Medicine, Northwestern University, Chicago, IL, United States, 3Department of Medical Imaging, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States, 4Department of Pediatrics, Division of Pediatric Cardiology, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States, 5Department of Pediatrics, Feinberg School of Medicine, Northwestern University, Chicago, IL, United States, 6Department of Biomedical Engineering, McCormick School of Engineering, Northwestern University, Chicago, IL, United States
Synopsis
The purpose of this study was to assess regional biventricular myocardial function in pediatric patients with repaired tetralogy of Fallot (TOF) by tissue phase mapping (TPM). Segmental left (LV) and right ventricular (RV) peak velocities were calculated in systole and diastole based on an extended AHA 16+10-segment model. Inter-ventricular dyssynchrony was quantified from the correlation between global LV and RV radial, long-axis and circumferential velocity time courses. Compared to age-matched healthy controls, TOF patients exhibited reduced long-axis peak velocities in systole and diastole as well as increased inter-ventricular dyssynchrony for radial and circumferential myocardial motion.
Introduction
Tetralogy
of Fallot (TOF)
is the most common cyanotic congenital heart disease, which is usually
surgically repaired during the first year of life. However, residual anatomic
and functional abnormalities are very common requiring life-long surveillance
and further treatment.1-4 Some of the most frequent abnormalities are
right ventricular (RV) dilation and dysfunction from pulmonary regurgitation as
well as left ventricular (LV) dysfunction. Standard imaging assessments4 based on simplified global parameters5-7 may not reflect regional myocardial abnormalities
or ventricular interactions. A more comprehensive evaluation of regional motion
and biventricular interaction might be beneficial. In
this context, tissue phase mapping (TPM) is
a well-established technique to quantify regional myocardial velocities,8-13 but has only recently been applied in children.14-16 The goal of this study was to test the
feasibility of TPM in pediatric patients with repaired tetralogy of Fallot to
quantify regional LV/RV myocardial velocities and to obtain biventricular
functional information.Methods
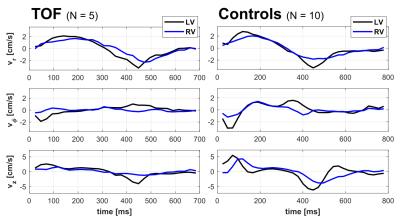
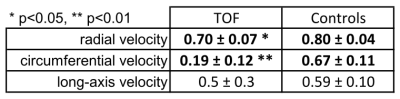
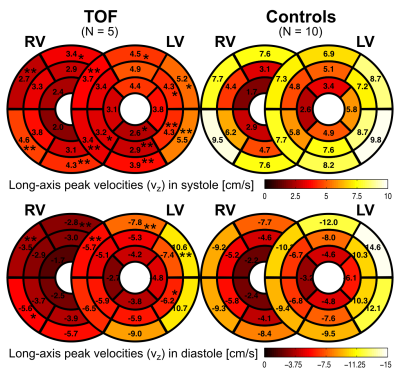
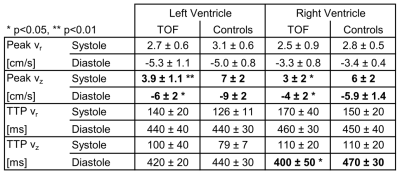
Five pediatric TOF patients (13+/-3y, 3f) and ten age-matched controls (15+/-3y [p=0.11], 6f) underwent standard CMR (1.5T Siemens Aera) including k-t accelerated (R=5) TPM using a black-blood prepared phase-contrast sequence with 3-directional velocity encoding8,10,17 in short-axis orientation (base, mid, apex; each in one breath-hold; in-plane resolution = (1.5-2.4 mm)2; temporal resolution = 21-25 ms; flip angle = 10° or 15°; venc = 25 cm/s in all directions). A single observer contoured LV and RV endo-/epicardial borders for calculation of time-resolved radial (vr), circumferential (vΦ), and long-axis (vz) LV/RV velocities over the cardiac cycle. For all components and slices pixelwise velocities (Fig. 1) were averaged over each ventricle to obtain global velocity time courses (Fig. 2). To assess the synchrony/dyssynchrony between LV and RV function, the cross-correlation coefficient (cc) between global LV and RV velocity time courses (i.e. averaged over base, mid, apex) was calculated for all 3 velocity components. The resulting cc is a measure of inter-ventricular dyssynchrony (cc=1: completely synchronous LV and RV motion, reduced cc = increased dyssynchony). For regional analysis pixelwise radial and long-axis velocities were also mapped onto an extended AHA model (16 segments for LV, 10 segments for RV,11 Fig. 3) to obtain segmental velocity time courses. Systolic and diastolic peak velocities and times to peak (TTP) velocity were calculated for each segment.Results
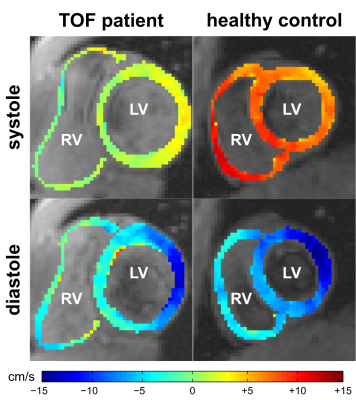
Figure 1 depicts systolic and diastolic LV/RV long-axis velocities in a TOF patient compared to a healthy control showing reduced velocities in the patient. Global velocity time courses for LV and RV appeared more synchronous for controls than patients (Fig. 2). This inter-ventricular synchrony was quantitatively assessed by calculating correlation coefficients, which were significantly reduced in patients for radial and circumferential velocities (Tab. 1). Figure 3 shows results for the regional analysis with reduced long-axis peak velocities in patients in systole and diastole. Considering global values (Tab. 2), radial peak velocities as well as radial and long-axis TTPs were similar, but LV/RV long-axis peak velocities were significantly reduced in TOF patients compared to controls.Discussion and Conclusion
Our pilot study shows that biventricular TPM analysis is feasible in pediatric TOF patients and demonstrates significant differences in regional ventricular function compared to control subjects. TOF patients exhibited reduced long-axis peak velocities, consistent with previous studies in young adults,12,13 and increased inter-ventricular dyssynchrony for radial and circumferential velocities. Larger studies are warranted to further characterize regional cardiac function in pediatric TOF patients and to potentially correlate these findings with clinical outcomes.Acknowledgements
Grant support by the National Institute of Heart, Lung and Blood Disorders (NHLBI) R01 HL 117888.References
1. Helbing WA, de Roos A. Clinical Applications of Cardiac Magnetic Resonance Imaging After Repair of Tetralogy of Fallot. Pediatr Cardiol. 2000 Jan 19;21:70–9.
2. Geva T. Repaired tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. J Cardiovasc Magn Reson. 2011;13:9.
3. Bonello B, Kilner PJ. Review of the role of cardiovascular magnetic resonance in congenital heart disease, with a focus on right ventricle assessment. Arch Cardiovasc Dis. 2012;105:605–13.
4. Valente AM, Cook S, Festa P, Ko HH, Krishnamurthy R, Taylor AM, et al. Multimodality Imaging Guidelines for Patients with Repaired Tetralogy of Fallot: A Report from the American Society of Echocardiography: Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance and the Society for Pediatric Radiol. J Am Soc Echocardiogr. 2014;27:111–41.
5. Davlouros PA, Kilner PJ, Hornung TS, Li W, Francis JM, Moon JCC, et al. Right Ventricular Function in Adults with Repaired Tetralogy of Fallot Assessed With Cardiovascular Magnetic Resonance Imaging: Detrimental Role of Right Ventricular Outflow Aneurysms or Akinesia and Adverse Right-to-Left Ventricular Interaction. J Am Coll Cardiol. 2002;40:2044–52.
6. Oosterhof T, Tulevski II, Vliegen HW, Spijkerboer AM, Mulder BJM. Effects of Volume and/or Pressure Overload Secondary to Congenital Heart Disease (Tetralogy of Fallot or Pulmonary Stenosis) on Right Ventricular Function Using Cardiovascular Magnetic Resonance and B-Type Natriuretic Peptide Levels. Am J Cardiol. 2006;97:1051–5.
7. Yoo BW, Kim JO, Kim YJ, Choi JY, Park HK, Park YH, et al. Impact of pressure load caused by right ventricular outflow tract obstruction on right ventricular volume overload in patients with repaired tetralogy of Fallot. J Thorac Cardiovasc Surg. 2012;143:1299–304.
8. Hennig J, Schneider B, Peschl S, Markl M, Laubenberger TKJ. Analysis of Myocardial Motion Based on Velocity Measurements with a Black Blood Prepared Segmented Gradient-Echo Sequence: Methodology and Applications to Normal Volunteers and Patients. J Magn Reson Imaging. 1998;8:868–77.
9. Jung B, Föll D, Böttler P, Petersen S, Hennig J, Markl M. Detailed Analysis of Myocardial Motion in Volunteers and Patients Using High-Temporal-Resolution MR Tissue Phase Mapping. J Magn Reson Imaging. 2006;24:1033–9.
10. Markl M, Rustogi R, Galizia M, Goyal A, Collins J, Usman A, et al. Myocardial T2-Mapping and Velocity Mapping: Changes in Regional Left Ventricular Structure and Function after Heart Transplantation. Magn Reson Med. 2013;70:517–26.
11. Menza M, Jung B, Komancsek A, Snyder J, Föll D. Segmental Tissue Phase Mapping analysis of biventricular heart function. In: Proc Intl Soc Mag Reson Med 21. 2013. p. 4483.
12. Chang M-C, Wu M-T, Menza M, Su M-Y, Huang H-C, Peng H-H. Evaluate Radial and Longitudinal Myocardial Motion Velocity in Left and Right Ventricles for Repaired Tetralogy of Fallot Patients by Phase-Contrast MRI. In: Proc Intl Soc Mag Reson Med 23. 2015. p. 2700.
13. Chang M-C, Wu M-T, Weng K-P, Su M-Y, Menza M, Huang H-C, et al. Left ventricular regional myocardial motion and twist function in repaired tetralogy of Fallot evaluated by magnetic resonance tissue phase mapping. Eur Radiol. 2017;doi:10.1007/s00330-017-4908-7.
14. Camarda J, Magrath P, Parekh K, Chowdhary V, Rigsby CK, Markl M. Co-registered MR tissue phase mapping and speckle tracking echocardiography: Inter-modality comparison of regional myocardial velocities in pediatric patients. J Cardiovasc Magn Reson. 2015;17(Suppl 1):1–2.
15. Ruh A, Lin K, Rose MJ, Robinson JD, Rigsby CK, Carr J, et al. Tissue Phase Mapping for Assessment of Biventricular Myocardial Motion in Children and Adults. In: ISMRM Workshop on Magnetic Resonance Imaging of Cardiac Function. New York, NY; 2017.
16. Gimpel C, Jung BA, Jung S, Brado J, Schwendinger D, Burkhardt B, et al. Magnetic resonance tissue phase mapping demonstrates altered left ventricular diastolic function in children with chronic kidney disease. Pediatr Radiol. 2017;47:169–77.
17. Jung B, Honal M, Ullmann P, Hennig J, Markl M. Highly k-t-Space-Accelerated Phase-Contrast MRI. Magn Reson Med. 2008;60:1169–77.
Figures