3315
Temporal changes in cardiac contractility, perfusion and infarct size after human cardiomyocyte transplantation to the infarcted heart of non-human primates.1Radiology, University of Washington, Seattle, WA, United States, 2Center for Cardiovascular Biology, University of Washington, Seattle, WA, United States, 3Institute for Stem Cell and Regenerative Medicine, University of Washington, Seattle, WA, United States, 4Department of Internal Medicine, National Cheng Kung University, Tainan, Taiwan, 5Pathology, University of Washington, Seattle, WA, United States, 6Medicine/Cardiology, University of Washington, Seattle, WA, United States
Synopsis
This study demonstrates structural and functional benefits of human embryonic stem cell derived cardiomyocyte engraftment to the infarcted heart of nonhuman primates. Those benefits were manifested in improved global and regional left ventricle contractile function, improved myocardial perfusion, decrease in infarct size and partial regeneration of the scarred myocardium. The benefit from human cardiomyocyte therapy was durable with the potential for further improvement in function between 1 and 3 months. The functional recovery was larger than were observed previously on small animal models of myocardial infarction, which might be due to the greater physiological match between human and macaque species.
INTRODUCTION
Nonhuman primates (NHPs) are one of the preferred large animal species for pre-clinical evaluations of therapeutic interventions due to their physiological, metabolic, and biochemical similarity to humans. Previously, we showed that transplantation of human embryonic stem cell derived cardiomyocytes (hESC-CMs) after myocardial infarction (MI) in NHPs leads to remuscularization and neovascularisation 1. The extent of restoration of the contractile function of the infarcted heart after hESC-CM transplantation is unknown. In the present study we used cardiac MRI techniques to evaluate changes in cardiac contractile function, myocardial perfusion and infarct size at one and three months after transplantation of hESC-CMs.
METHODS
Myocardial infarction was induced in Macaca nemestrina (10±2 kg, 9±2 years old, both sexes) by 3 hour mid-LAD balloon inflation followed by reperfusion. 14 days post-infarction, 750 million of hESC-CMs were directly injected into the infarct region and boarder zones. In vivo MRI studies were conducted in 4 control and 4 cell-treated NHPs using a 3T clinical scanner (Achieva Philips, Best, Netherlands) one day prior and 27 days after cell delivery. One control and one treated animal were followed up to 3 months. MRI protocol included following steps: 1) CINE images to assess left ventricle (LV) geometry and contractility; 2) For evaluation of myocardial perfusion, dynamic scans were acquired using a single-shot saturation recovery T1-TFE sequence. During the acquisition, 0.2 mmol/kg ProHance (Bracco Diagnostics Inc., Princeton, NJ) bolus was injected intravenously followed by a saline flush. 3) To visualize infarct, phase-sensitive inversion recovery (PSIR) multislice images were acquired at 8 minutes after the contrast agent injection with inversion time range 280-350 msec. Perfusion analysis was performed using a discrete-time model that estimates the transfer constant (Ktrans) that describes the uptake of contrast agent into the tissue, the contrast retention coefficient (R) 2.RESULTS
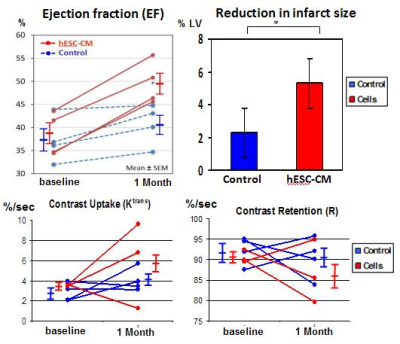
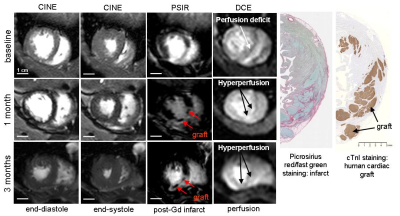
Prior to treatment, hearts of NHPs exhibited depressed LV ejection fraction (EF), averaging 38.1±4.1% (mean±St.Dev), absence of LV wall motion in the infarcted zone and large myocardial infarction averaging 20±6% to LV area. Contractile function of the infarcted heart substantially improved after cell transplantation in comparison with no-cell control infarcted animal. Specifically, EF in cell treated animals was 50±5% in comparison with untreated control (40±4%, p=0.01); we also observed increased infarcted wall motion (23±15% of the LV wall, p=0.05), and trend to increase in myocardial perfusion (increase in Ktrans, decrease in contrast retention, figure 1). One animal was excluded from the perfusion analysis due to failed cardiac triggering during injection. Another cell-treated animal showed decrease in Ktrans from 3.62 (baseline) to 1.29 (1 month); it correlates with the histology data showing loss of human graft. Large areas of a new viable myocardium were detected inside the scar zone in three out of four cell-treated animals at one month after hESC-CM transplantation (figure 2). Scar shrinkage was more pronounced in cell-treated NHPs by one month after cell transplantation (5.3 ± 0.2% vs. 2.3 ± 0.9% in control animals, p<0.05, figure 1). The functional benefits were durable up to 3 months post-engraftment: the EF improved from 51% at day 27 to 66% at 3 months in cell-treated animal (figure 2); the EF decreased in control animal from 43.9% at day 27 to 40.4% at 3 months.DISCUSSION AND CONCLUSION
This study demonstrates structural and functional benefits of hESC-CM engraftment to the infarcted heart of NHPs. Those benefits were manifested in improved global and regional LV contractile function, improved perfusion and decrease in infarct size as well as partial regeneration of the scarred myocardium. Increase in Ktrans, decrease in contrast retention might be related to neovasculature in graft area, as we have shown previously in heart of non-human primates 1, or might be related to viable mass increase (figure 2). The benefit from hESC-CM therapy appears to be durable with the potential for further improvement in function between 1 and 3 months. The functional recovery was larger than were observed previously on small animal models of myocardial infarction 3,4, which might be due to the greater physiological match between human and macaque species.Acknowledgements
These studies were supported in part by NIH Grants R01 HL084642, P01HL094374, and a grant from the Fondation Leducq Transatlantic Network of Excellence (all to CEM). These studies also were supported by the UW Medicine Heart Regeneration Program, the Washington Research Foundation, and the Garvey Family Foundation.References
1. Chong JJ, Yang X, Don C, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014;510(7504):273-277.
2. Kerwin WS, Naumova A, Storb R, et al. Mapping contrast agent uptake and retention in MRI studies of myocardial perfusion: case control study of dogs with Duchenne muscular dystrophy. Int J Cardiovasc Imaging 2013;29(4):819-826.
3. Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol 2007;25:1015-1024.
4. Shiba Y, Fernandes S, Zhu WZ, et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 2012;489:322-325.
Figures