3310
MRI of whole brain formalin-fixed samples at 9.4T: influence of the fixation agent and its dielectric properties on image qualityGisela E Hagberg1,2, Thomas Shiozawa-Bayer3, Christian Mirkes2, Jörn Engelmann2, Jonas Bause2, Bernhard Hirt3, and Klaus Scheffler1,2
1Biomedical Magnetic Resonnce, University Hospital Tübingen, Tübingen, Germany, 2High Field Magnetic Resonance, Max Planck Institute for Biological Cybernetics, Tübingen, Germany, 3Institute of Clinical Anatomy, University Hospital Tübingen, Tübingen, Germany
Synopsis
MRI of post mortem samples in formalin is an essential tool for validation purposes and comparison with clinical histology, since fixation preserve several microstructural tissue features. We found that the dielectric properties of the fixative influences image quality attained in whole brain post mortem samples at 9.4T. The standard fixative prevented high quality MRI across the entire sample. By using a high conductivity fixative with less field focussing, a more homogeneous excitation was achieved without any drop outs and T1 mapping could be performed using rapid inversion recovery techniques.
Introduction
MRI of post mortem samples is essential for validation purposes and comparison with clinical histology, since fixation preserves several microstructural tissue features [1-3]. At high magnetic fields, the dielectric properties are determining for reducing the dielectric imaging artifact [4-5] and are key to achieve homogeneous spin excitation and prevent tissue heating. In the present study we investigate the influence of the dielectric properties of different fixatives on MRI image quality for whole brain imaging of post-mortem samples using clinical mesurement protocols.Materials&Methods
MRI of tissue samples and solutions was performed at 9.4T with a 16ch transmit(Tx) /31ch receive (Rx) array [6] operating in CP mode. A 2.8L cylindrical container, filled with either of 2 different paraformaldehyde based fixatives (FIX2 and FIX3), phosphate buffer (PBS), or ethanol (99% UN1107 denaturated with MEK/Bitrex) was scanned. The 3D transmit field was mapped with the Actual Flip Angle method [7] using a nominal flip angle of 60°; TR1/TR2=20/100ms; TE=7ms, voxel size=3x3x5mm³; TA=3min45s. The reference voltage was set to achieve the nominal flip angle at the maximum of the CP-mode sweet-spot, located at the center of the container. T1 relaxometry was performed by an inversion-recovery echo-planar imaging (IE-EPI) sequence at TI=50, 100, 150, 200, 250, 350, 450, 650, 850, 1100, 1500, 2000, 3000, 4000, 6000ms; with TE=11ms; TR=11s; voxel size of 2x2x2mm³; partial Fourier factor=6/8; GRAPPA=3; TA=3min45s. Three whole brain post-mortem samples (obtained through the local body donor program) were kept in two formalin-based fixatives (FIX1, similar to FIX2, and FIX3) for a minimum of four months prior to MRI scanning. The brain samples occupied ca 1.2 Liter; >40% of the container. For comparison, a smaller (1.5L) half-dome shaped container, in which the brain sample occupied >66% of the total volume, was also used. Besides the AFI and T1mapping used for the solutions, whole brain T1 mapping was performed with the MP2RAGE sequence based on an optimized 13ms long non-selective TRFOCI pulse for inversion (TI1/TI2=900/3500ms; FA=4/6°; read-out TR =6ms; inversion TR=8894ms, 0.8mm isotropic voxel size; TA=9min40s). The dielectric properties of the different solutions (FIX1, FIX2, FIX3, PBS, Ethanol) were measured at room temperature using a SPEAG (Zürich, Switzerland) dielectric assessment kit (DAK-12 probe) and an Agilent Technologies E5071 network analyzer (Santa Clara, United States), across the frequency range 100-600MHz in steps of 1MHz. Likewise, the permittivity and the conductivity of a sucrose-based solution, mimicking the dielectric properties of the human brain at 400MHz was measured [5].Results&Discussion
The permittivity of each fixative was similar to PBS but was substantially greater than the tissue mimicking solution and ethanol, while the conductivity was generally higher (Fig1 with Table). Although ethanol is a good fixative and has low permittivity, it yields unwanted, shifted ghost images owing to its multiplet resonance spectra. An acceptable trade-off with slightly lower permittivity and greater conductivity was achieved by FIX 3, albeit at the cost of an increased coil-load and a higher voltage required to reach the nominal flip angle. The scaling factor between the actual and nominal flip angle reached minimal values with FIX 2, while with both FIX 3 and PBS the actual field was always at least 35% of the nominal values (Fig.2A). In accordance, it was not possible to properly invert the spins with FIX 2, while no such unsurmountable issues were observed with either FIX3 or PBS (Fig 2B). Likewise, an insufficient inversion was observed in the brain sample containing the low-conductivity fixative (FIX1). Reducing the size of the container did not alleviate this issue, likely because the size of the brain is greater than the RF wavelength in this fixative. Conversely, the use of the high conductivity fixative FIX3 was sufficient to alleviate this issue and a homogeneous inversion field could be attained (Fig 3). These observations are consistent with the stronger field focusing occurring in conditions of low conductivity [4].Conclusion
For whole brain post mortem MRI studies performed at 9.4T, the choice of fixative determines the extent of B1 field focusing, and hereby the image quality that can be achieved.Acknowledgements
Funding by the Max Planck Society, and the ministry of Science, Research and the Arts of Baden-Württemberg (Az: 32-771-8-1504.12/1/1)References
[1] Bagnato F et al. Brain. 2011 ;134(Pt 12):3602-15. [2] Foxley S et al., Neuroimage. 2014;102 Pt 2:579-89. [3]Weiss M, et al., Brain Struct Funct. 2015;220(3):1695-703. [4] Sengupta S et al., Neuroimage. 2017 in press [4] Hoult DI & Phil D J Magn Reson Imaging. 2000;12(1):46-66 [5] Hoffmann J et al., MAGMA. 2014;27(5):373-86 [6] Shajan, G., et al., (2013). Magn Reson Med;71(2):870-9 [7] Avdievich NI et al. Magn Reson Med. 2016;76(5):1621-1628. [8] Yarnykh VL. Magn Reson Med 2007;57(1):192-200Figures
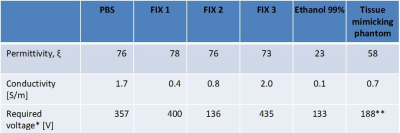
Table: Dielectric properties of fixatives and solutions at 400MHz,
corresponding to the Larmor frequency of MRI scanners operating at 9.4T, and voltage required to reach the nominal angle in the center of a 2.8L container in a
16/31Tx/Rx coil [6] **value measured in a different, head-shaped
container
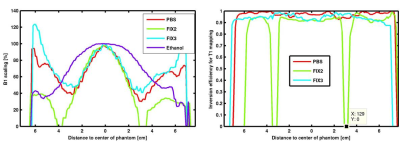
A) Scaling factors between the nominal and the
actual flip angle played out and B) the ensuing inversion efficiency observed in
a 2.8L container filled with the different solutions and measured at 9.4T. For ethanol
multiple resonances impeded proper mapping of the inversion efficiency, althoug flip angle mapping was possible.
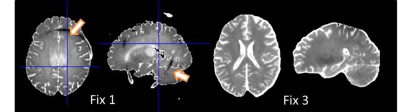
Whole brain MRI of two post mortem samples in different fixatives (low conductivity Fix 1, and
high conductivity Fix 3). T1 maps obtained by
MP2RAGE are shown. The different dielectric properties lead to
differences in the actual flip angle played out. Drop outs in areas with insufficient spin
inversion (see arrows in frontal and posterior parts of the brain) occurred using
Fix 1. These issues could be alleviated by the use of Fix 3.