3305
Ultrafast 7T EEG-fMRI for epilepsy using 3D paradigm-free models1Imaging Institute, Cleveland Clinic Foundation, Cleveland, OH, United States, 2Neurologic Institute, Cleveland Clinic Foundation, Cleveland, OH, United States, 33Basque Center of Cognition, Brain and Language, San Sabastian, Spain
Synopsis
There is enormous benefit for non-invasive MRI techniques guiding neurosurgeons to resect tissue causing epilepsy. We extend traditional EEG-fMRI methods in 3 ways: apply 7T to increase BOLD signal; use increased temporal resolution (TR 300ms) from multiband techniques to separate primary from secondary epileptogenic zones; and use paradigm-free mapping to identify interictal spikes obtained during long scans. We test this methodology using isolated finger taps as a surrogate for epileptogenic spikes. Close correspondence between conventional event analysis and paradigm free mapping suggests epileptogenic spikes can be reliably detected if their HRF is similar to a single finger tap.
Introduction
Patients suffering from intractable focal epilepsy can be cured if the epileptogenic zone (EZ) is identified and resected. While EEG and MRI are often helpful to identify this zone, many patients remain untreated due to the failure of these techniques. Thus there is a need for more advanced techniques to identify the EZ.
One promising technique is simultaneous EEG-fMRI, wherein epileptogenic spikes are identified by their characteristic BOLD effects during an EPI acquisition. Classically this technique requires simultaneous EEG to identify the time-points of spikes, so the BOLD analysis can retrospectively perform an event-related analysis.
We propose to extend existing progress in this field in 3 ways: to use 7T to increase the SNR and BOLD contrast; to use multiband techniques to reduce the TR to 300 ms and thereby attempt to visualize the temporal evolution of spikes; and to analyze the data using paradigm free mapping, an approach that is data-driven and does not required an epileptologist to examine an hour of EEG data for a few spikes. Prior to testing on epilepsy patients, in this work the methods is tested on a normal human subject using single finger taps to an audible command as a surrogate for an epileptogenic spike.
Methods
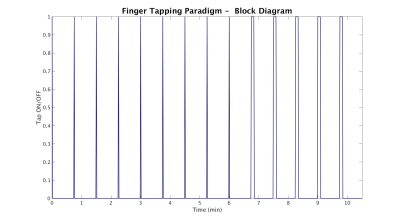
One healthy control subject was scanned twice on different days at 7T (Siemens) using a 32-channel receive transmit coil (QED, Cleveland, OH). A volumetric MP2RAGE1 was acquired for anatomy. On the first day, the protocol consisted of a simultaneously multi-slice excited (SMS) EPI collected at TR=2800ms (1.2x1.2x1.5mm, MB factor=3) and 500ms (iso-3mm, MB factor=3). On the second day, EPI data was collected at TR=500ms (iso-3mm, MB factor=3) and 300ms (iso-3mm, MB factor=4). During scanning, finger tapping was performed in a block designed ON and OFF paradigm with 45 second cycles (see Figure 1). For the first 6 minutes, a single finger tapping from the right index finger to the right thumb was performed. For the remaining 4 minutes, the subject was instructed to repeat the same finger tapping sequentially 10 times.
Data were analyzed in two different ways. A standard GLM analysis was performed with the tapping categorized as events. Additionally, a data driven, paradigm-free method was employed2. Data were first corrected for motion using SLOMOCO3 and then detrended using 3dDetrend. Brain extraction was performed to create a mask using FSL bet. 3dPFM was executed using the LASSO algorithm along with the Bayesian Information Criterion for deconvolution, and the SPM canonical HRF was used as the hemodynamic model. Finally, these same techniques were applied to data from 12 epilepsy patients where EEG-fMRI was simultaneously collected using the same protocol as day 1 of the finger tapping.
Results and Discussion
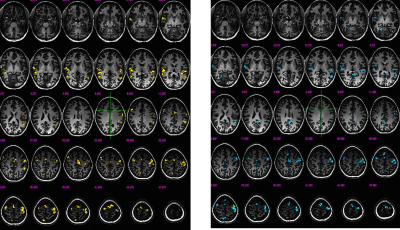
BOLD activation maps are readily generated using 7T EEG-fMRI techniques using single finger taps as a surrogate for epileptogenic spikes, either with models using known time-points, or data-driven techniques using paradigm free models. BOLD maps are also readily generated at either TR=0.5 sec or TR=0.3 sec. Statistical parametric maps using these two independent methods generate similar maps (Figure 2), suggesting that a paradigm-free method could successfully detect epileptogenic spikes assuming the resulting BOLD HRF is similar to that assumed in the model, here the canonical HRF for a single finger tap. Early results applied to epilepsy patients support this hypothesis, as confirmed with correlations to MEG and post-operative outcome following resective surgery. Figure 3 shows the results from a BOLD map using TR=0.5 sec in the same region as the MEG results from the same epilepsy patient.Conclusion
This work advances current EEG-fMRI techniques for the evaluation of epilepsy patients in 3 ways: 7T for increased SNR and BOLD contrast; shorter TR (300 msec) using multiband techniques; and analysis using data-driven technique of paradigm-free mapping.Acknowledgements
This work was supported by Cleveland Clinic. Author gratefully acknowledges technical support by Siemens Medical Solutions, especially Dr. Tobias Kober for MP2RAGE, from Dr. Himanshu Bhat, for SMS EPI.References
1. Marques JP, Kober T, Krueger G, et al. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage. 2010;49(2):1271-1281.
2. Caballero Gaudes C, Petridou N, Francis ST, Dryden IL, Gowland PA. (2013). Paradigm free mapping with sparse regression automatically detects single-trial functional magnetic resonance imaging blood oxygenation level dependent responses. Hum. Brain Mapp. 34(3), 501-518.
3. Beall EB, Lowe MJ. SimPACE: generating simulated motion corrupted BOLD data with synthetic-navigated acquisition for the development and evaluation of SLOMOCO: a new, highly effective slicewise motion correction. Neuroimage. 2014;101:21-34.
Figures