3304
Concurrent changes in cerebral temperature, lactate and vasomotion in urethane anesthetized rats. Proton magnetic resonance study at 7T.1Psychology, Sheffield University, Sheffield, United Kingdom, 2Biological Sciences, Sheffield University, Sheffield, United Kingdom, 3School of Cancer Sciences, University of Birmingham, Birmingham, United Kingdom
Synopsis
Cerebral vasomotion is frequently observed phenomenon that accompanies hypotension (mean arterial blood pressure (MABP) 45-65mmHg) in anesthetised rat models(1,2). Although unclear, this mechanism appears to play, in part, a compensatory role in replenishing oxygen reserves in the anaerobic/hypoxic brain. Although the relationship between temperature and metabolism is always interactive. Brain cell metabolism is a major determinant of brain temperature, minor changes in brain temperature can result in significant changes in neural cell metabolism and therefore in brain function. During hypotension (MABP 45-65mmHg) analysis of acquired 1H-spectra revealed thalamic temperature to be ~1.5-2 °C colder than that of the core body temperature (37±0.5)°C. Further analysis of the 1H-spectra revealed dynamic pool of the lactate in the thalamus during hypotension (MABP 45-65mmHg).
Introduction
Neurovascular coupling matches local blood flow to the metabolic demand of brain tissue, and also supports removal of metabolic byproducts. This requires proper regulation of vascular tone of cerebral micro vessels, which then support the stable flow of nutrient rich blood to glial cells, thereby facilitating normal neuronal function and brain metabolism (3,4). However, with ageing sporadic ischemic insults likely deteriorate brain tissue perfusion and local tissue oxygenation in both human and animal models of human cerebrovascular pathologies (5,6). Unstable micro vascular flow and depleted oxygen reservoirs trigger spontaneous rhythmic variations in the vessel lumen as a result of smooth muscle constriction and dilatation. This rhythmic sinusoidal low frequency (0.1 Hz) oscillation is termed as vasomotion (1,2).Cerebral vasomotion has been reported in several species including the rat (1), awake rabbits (7), and recently in the brain of patients undergoing surgical resection of a brain tumour (8). Despite extensive research efforts, understanding of the underlying mechanisms and the functional implications of vasomotion remains poorly understood. Therefore, non-invasive physiological measurement of cerebral vasomotion and its effect on cerebral metabolism is needed for accurate assessment of its functional significance.Method
We study effects of pre and post phenylephrine infusion on the brains of adult (female-Hooded Lister) rats (weighing 240-340gm). Animals were prepared for MRI experiments as described previously (9,10). A series of 1H PRESS (TE=24, & 134ms, TR=2.5s, SWH=5000Hz, voxel 56ul), and GE-EPI time series data (Matrix=64x64, FOV=3x2, slices=9, TR=1, TE=12ms, Repetitions=1800, flip angle=90) were acquired at 7T (Bruker, Biospec, 30cm horizontal bore) as described before (9,10). MR data analyses were carried out using Bruker image and spectra processing toolboxes in complement with python based VESPA, JMRUI toolboxes. Metabolite ratios and temperature data were statistically compared in Graphpad prism. In-vitro temperature calibrations were done on a Glycine/water (1.5-2mM glycine dissolved in saline filled in a 50 ml Vulcan tube) phantom, and in-vivo rat brain temperature were obtained as described before (11,12).Results
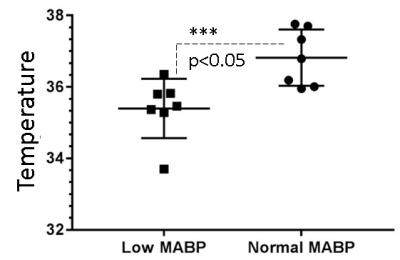
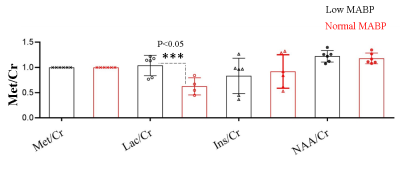
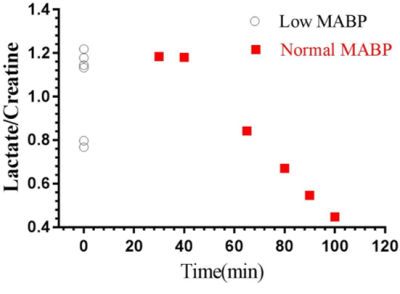
We measured neurochemical markers in the deep brain (thalamus) of urethane anesthetized rat pre-and post-phenylephrine infusion. First condition prior to phenylephrine infusion vasomotion remained active and mean arterial blood pressure (MABP) stayed low (45-65 mmHg). While the second condition, continuous phenylephrine (>30-180 mins) post-infusion, and forced respiration at (60-85 breaths/min) restored normal mean arterial blood pressure (110±20 mmHg), and vasomotion was suppressed. Under low MABP (45-65 mmHg, pre-phenylephrine infusion), and normal MABP (110±20mmHg, post-phenylephrine infusion) deep brain temperature in the rat thalamus were 35.4±0.8 °C and 36.8±0.8 °C respectively (see figure 1). Thus in the anesthetized rat thalamic temperature was 1.5-2 °C colder than that of the core body temperature (37±0.5) °C. Suggesting deep brain temperature in rodents can be therapeutically tuned and monitored by continuous infusion of phenylephrine. Accurate estimation of brain temperature may potentially permit indirect but subtle measurement of fluctuations affecting the BOLD signal as explained before (13-15). Figure 2 provides an explanation for the altered metabolic status of the hypo-perfused brain subjected to 0.1 Hz continuous oscillations. At low MABP increased cerebral Lac/Cr temperature may potentially permit indirect but subtle measurement of fluctuations affecting the BOLD ratio was significantly augmented compared with Lac/Cr ratio at normal MABP. Figure 3 shows only a tiny fraction of lactate can be reliably detected after ~90-100 mins post-phenylephrine infusion. These two results clarify indirect association between vasomotion and lactate retention in the brain. Furthermore, long-TE-1H-PRESS (TE=134ms, data not shown) spectra unambiguously confirm the build-up lactate in the hypo-perfused brain oscillating with a large amplitude and at a low frequency (0.1Hz).Discussion
Cerebral vasomotion is frequently observed phenomenon that accompanies hypotension (mean arterial blood pressure (MABP) 45-65mmHg) in anesthetised rat models(1,2). Although unclear, this mechanism appears to play, in part, a compensatory role in replenishing oxygen reserves in the anaerobic/hypoxic brain. Although the relationship between temperature and metabolism is always interactive. Brain cell metabolism is a major determinant of brain temperature, minor changes in brain temperature can result in significant changes in neural cell metabolism and therefore in brain function. During hypotension (MABP 45-65mmHg) analysis of acquired 1H-spectra revealed thalamic temperature to be ~1.5-2 °C colder than that of the core body temperature (37±0.5)°C. Further analysis of the same 1H-spectra revealed dynamic pool of the lactate in the thalamus during hypotension (MABP 45-65mmHg). Our findings suggest lactate in the brain can be measured by 1H MRS because the tissue clearance of lactate potentially remains slower than the active build-up of lactate in the thalamic tissue. In future studies we shall investigate potential consequences of a large amplitude of Vaso-signal and its impact on the cerebral lactate clearance via glial cells.
Acknowledgements
No acknowledgement found.References
1. Mayhew, J.E., Askew, S., Zheng, Y., Porrill, J., Westby, G.W., Redgrave, P., Rector, D.M. and Harper, R.M. (1996) Cerebral vasomotion: a 0.1-Hz oscillation in reflected light imaging of neural activity. NeuroImage, 4, 183-193. 2. Kennerley, A.J., Harris, S., Bruyns-Haylett, M., Boorman, L., Zheng, Y., Jones, M. and Berwick, J. (2012) Early and late stimulus-evoked cortical hemodynamic responses provide insight into the neurogenic nature of neurovascular coupling. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 32, 468-480. 3. Paulson, O.B., Strandgaard, S. and Edvinsson, L. (1990) Cerebral autoregulation. Cerebrovascular and brain metabolism reviews, 2, 161-192. 4. Zlokovic, B.V. (2011) Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nature reviews. Neuroscience, 12, 723-738. 5. Intaglietta, M. (1991) Arteriolar vasomotion: implications for tissue ischemia. Blood vessels, 28 Suppl 1, 1-7. 6. Brown, W.R. and Thore, C.R. (2011) Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathology and applied neurobiology, 37, 56-74. 7. Hundley, W.G., Renaldo, G.J., Levasseur, J.E. and Kontos, H.A. (1988) Vasomotion in cerebral microcirculation of awake rabbits. The American journal of physiology, 254, H67-71. 8. Rayshubskiy, A., Wojtasiewicz, T.J., Mikell, C.B., Bouchard, M.B., Timerman, D., Youngerman, B.E., McGovern, R.A., Otten, M.L., Canoll, P., McKhann, G.M., 2nd et al. (2014) Direct, intraoperative observation of ~0.1 Hz hemodynamic oscillations in awake human cortex: implications for fMRI. NeuroImage, 87, 323-331. 9. Berwick, J., Johnston, D., Jones, M., Martindale, J., Redgrave, P., McLoughlin, N., Schiessl, I. and Mayhew, J.E. (2005) Neurovascular coupling investigated with two-dimensional optical imaging spectroscopy in rat whisker barrel cortex. The European journal of neuroscience, 22, 1655-1666. 10. Kennerley, A.J., Berwick, J., Martindale, J., Johnston, D., Papadakis, N. and Mayhew, J.E. (2005) Concurrent fMRI and optical measures for the investigation of the hemodynamic response function. Magnetic resonance in medicine, 54, 354-365. 11. Zhu, M., Bashir, A., Ackerman, J.J. and Yablonskiy, D.A. (2008) Improved calibration technique for in vivo proton MRS thermometry for brain temperature measurement. Magnetic resonance in medicine, 60, 536-541. 12. Babourina-Brooks, B., Simpson, R., Arvanitis, T.N., Machin, G., Peet, A.C. and Davies, N.P. (2015) MRS thermometry calibration at 3 T: effects of protein, ionic concentration and magnetic field strength. NMR in biomedicine, 28, 792-800. 13. Boorman, L., Kennerley, A.J., Johnston, D., Jones, M., Zheng, Y., Redgrave, P. and Berwick, J. (2010) Negative blood oxygen level dependence in the rat: a model for investigating the role of suppression in neurovascular coupling. The Journal of neuroscience : the official journal of the Society for Neuroscience, 30, 4285-4294. 14. Zheng, Y., Pan, Y., Harris, S., Billings, S., Coca, D., Berwick, J., Jones, M., Kennerley, A., Johnston, D., Martin, C. et al. (2010) A dynamic model of neurovascular coupling: implications for blood vessel dilation and constriction. NeuroImage, 52, 1135-1147. 15. Devonshire, I.M., Papadakis, N.G., Port, M., Berwick, J., Kennerley, A.J., Mayhew, J.E. and Overton, P.G. (2012) Neurovascular coupling is brain region-dependent. NeuroImage, 59, 1997-2006.Figures