3301
Localizing nociresponsive region within area 3a in human cortex using fMRI at 7T1Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom, 2Liverpool John Moores University, Liverpool, United Kingdom, 3University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
Synopsis
A component of Brodmann area 3a has been shown to be highly responsive to thermonoxious skin stimulation in monkey studies. Here, we use BOLD fMRI at 7T to map on a fine scale the functional representation of a noxious heat stimulus in human primary somatosensory cortex (S1), and compare this to the hand representation identified from vibrotactile stimulation. Thermonoxious stimulation of both the palm and digits evoked a spatially distinct activation within S1, which extends beyond and partially overlaps with the anterior area responding to vibrotactile activation, consistent with the location of nociresponsive area 3a in monkeys.
Introduction
Previous studies in squirrel monkeys have demonstrated that a part of Brodmann area 3a is highly responsive to thermonoxious skin stimulation [1] and further found that monkeys who have this region removed by surgery show permanently reduced pain sensibility [2]. In humans, the precise location of area 3a in the central sulcus varies extensively across individuals [3], and the involvement of area 3a in pain sensation has not been explored. Here, we aim to localize the nociresponsive subregion of area 3a in human somatosensory cortex using high-resolution fMRI at 7T.Methods
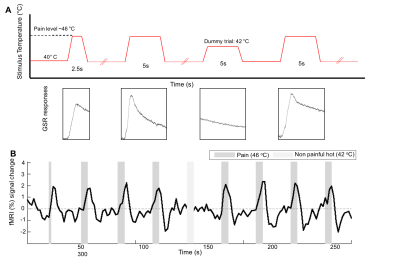
One subject was scanned on three separate occasions on a 7T Philips Achieva scanner using a 32-channel receive coil. fMRI data was collected during thermonoxious skin stimulation. Thermal painful stimuli were applied to either the palm or to the tips of digits 2 and 3 on the right hand, using an MRI-compatible Peltier thermode (Medoc Pathway). Prior to scanning, the subject’s pain threshold was determined for both the palm (46-46.5oC) and fingertips (48.5oC). In a fourth scan session, a travelling wave paradigm comprising of non-noxious vibrotactile stimulation was used to map the representation of the individual digits in the contralateral primary sensory cortex (S1) [4]. fMRI paradigm: The fMRI paradigm was a block design with 5s periods of stimulus interleaved with off periods (40oC baseline) of varying (25-30s) inter-stimulus interval (Fig. 1). Each fMRI run comprised 8 trials of thermonoxious stimulation and lasted 5minutes. In all three scan sessions, two pain fMRI runs were performed on the palm. In the 2nd session, an additional pain run was performed on the fingertips. In the 3rd session, two additional non-painful heat runs (at 42oC, with a baseline of 37oC) were performed on the palm. Galvanic skin responses (GSR) were collected throughout the thermal stimulation to monitor the degree of perceived pain. MR acquisition: fMRI data were acquired using a multi-slice gradient-echo EPI acquisition (TE=25ms, TR= 2s, FOV=192(AP)×164(RL)mm2, SENSE-factor=1.2(AP), half-scan=0.8) with multi-band factor of 3, allowing the acquisition of 54 slices at 1.5 mm isotropic resolution. A structural whole-head anatomical MPRAGE (1mm isotopic) was also collected for cortical unfolding. Analysis: The thermonoxious fMRI time series were analyzed using a GLM including the temporal derivative. Resulting z-score maps were corrected for multiple comparisons, and corrected for geometric distortions using FSL’s TOPUP command. Fourier analysis was applied to the travelling-wave data to generate a digit somatotopic (phase) map, this was threshold at a coherence>0.3. Cortical surface reconstructions were generated using Freesurfer. The location of the activations evoked by thermonoxious stimulations were spatially compared with the location of the hand-digit ROI from the vibrotactile paradigm and the subject-specific Freesurfer labels for Brodmann S1 areas.Results
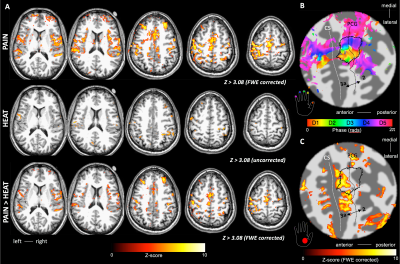
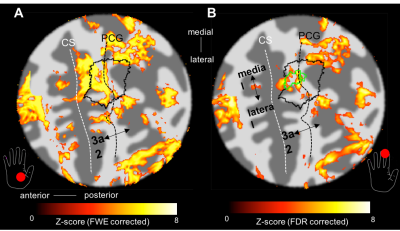
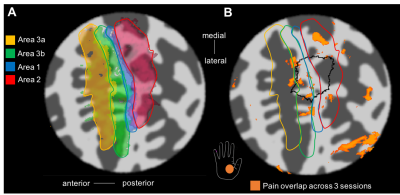
BOLD responses where highly modulated by the painful stimuli, with minimal activation to the heat (no pain) stimulus, in agreement with the GSR trace (Fig. 1B). Regions modulated by painful thermal stimulation of the palm include primary and secondary somatosensory cortices, as well as insula, anterior cingulate, and prefrontal cortices (Fig. 2A), all areas known to form components of the pain matrix [5]. Within contralateral S1, the contrast Pain>Heat for the palm stimulation yielded activation maps which partially overlap the hand ROI defined from the vibrotactile travelling wave, but this activation also extends more anterior and lateral (Fig. 2C and 3A). For the fingertip stimulation, responses within S1 were more localized to the hand ROI (Fig. 3B), but also extended anteriorly. Figure 4 shows the overlap of activations evoked by the painful palm stimulation across all three scan sessions, showing a region located in the depth of the central sulcus, consistent in the location with area 3a (Freesurfer labels).Discussion
Area 3a is primarily the part of S1 that represents deep receptors and musculature of the contralateral body. Here, we demonstrate a nociresponsive region within human S1 which lies anteriorly with respect to non-noxious responses to vibrotactile stimulation of the digits, consistent with the predicted location of area 3a. This region could potentially be targeted for surgery in patients suffering from chronic pain, making it a highly attractive means of treating chronic pain [1].Conclusion
Human responses to thermonoxious hand stimulation at 7T on the subject studied here are consistent with the response of monkey area 3a to the same type of stimulation [1]. We have shown that activation maps are robust and reproducible (overlap across 3 sessions for z>3.08 after FWE correction). Future work will include more subjects to corroborate this finding.Acknowledgements
This work was funded by a Leverhulme fellowship and MRC grant MR/M022722/1.References
1. Vierck CJ, Whitsel BL, Favorov OV, Brown AW, Tommerdahl M. The roles of primary somatosensory cortex in the coding of pain sensations. Pain. 2013; 154: 334-344.
2. Favorov OV, Challener T, Whitsel BL et al. Increasing the effectiveness of postcentral topectomy in chronic pain relief by targeted inactivation of nociresponsive area 3a. Soc for Neurosci Abstracts. 2017. Program No. 486.15. 2017 Neuroscience Meeting Planner. Washington, DC.
3. Geyer S, Schleicher A, Zilles K. Areas 3a, 3b and 1 of human primary somatosensory cortex. NeuroImage. 1999; 10:63-83.
4. Sanchez Panchuelo RM, Francis S, Bowtell R, Schluppeck D. Mapping human somatosensory cortex in individual subjects with 7T functional MRI. J Neurophysiology. 2010; 103:2544-2556.
5. Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron, 2007;55(3):377-391.
Figures