3299
Validation of a Radiological Definition for Central Vessel Sign using 7T FLAIR and SWI1Robarts Research Institute, Western University, London, ON, Canada, 2Graduate Program in Biomedical Engineering, Western University, London, ON, Canada, 3Medicine, Western University, London, ON, Canada, 4Department of Neurology/Neurosurgery, McGill University, Montreal, QC, Canada, 5Radiology and Clinical Neurological Sciences, Western University, London, ON, Canada, 6Department of Clinical Neurological Sciences, Western University, London, ON, Canada
Synopsis
A number of recent clinical radiology studies support the central vessel sign (CVS) as a sensitive and specific means of differentiating MS white matter lesions (WML) from non-MS WML. However, these studies have employed varying practices for imaging veins and WMLs. This has led to an inconsistent radiological definition of CVS. Recently, the North American Imaging in Multiple Sclerosis publisheda set of guidelines, which provide grounds for derivation of a more robust radiological definition of CVS. Here we employ these guidelines, together with FLAIR and SWI at high field to arrive at a sensitive and specific radiological definition of CVS.
Introduction
Multiple sclerosis is an autoimmune disorder diagnosed in the clinic using the expanded disability status scale. Using MRI, and specifically fluid attenuated inversion recover (FLAIR) images, MS can be diagnosed by visualizing the hyperintense white matter lesions (WML) on MRI images. However, WMLs alone are not specific to MS. The number of non-specific WMLs in brain white matter is known to increase with age and with certain risk factors in healthy subjects. Other imaging biomarkers have been proposed for differentiating MS from its mimics. One such marker is the central vessel sign (CVS)1. Identification of the CVS in MS white matter lesions has been shown in various MRI studies,2,3. However, these studies have employed a variety of different practices for visualizing the lesions and the vessels. A consensus statement was recently published by the North American Imaging in Multiple Sclerosis (NAIMS)4 consortium with the specific goal of achieving a more consistent radiological definition of CVS.
The role of susceptibility weighted imaging (SWI) in visualizing cerebral veins is well established. However, the quality of information obtained from SWI depends heavily on the quality of the associated phase images. Such images can suffer from phase wrap artifacts, which are exacerbated in high field MRI. While high-field imaging has shown to be more effective in some clinical applications,5 phase image processing is still very challenging at higher field strengths. This makes high field SWI less likely to be reliably adapted in the clinic. Recently an approach has been presented for high field SWI, based on channel-by-channel phase processing, followed by the application of robust channel-combination weighting using the inter-echo variance of the high-pass filtered phase images (IEV-SWI).6 IEV-SWI preserves phase information thereby enabling the definition of high quality venography images. In this study, we employed the IEV-SWI approach together with optimized magnetization prepared FLAIR (MP-FLAIR)7 images to define a sensitive and specific radiological definition of CVS.
Methods
The study was approved by our institutional REB. Written consent was obtained from all participants. 17 relapsing remitting MS (RRMS) patients and 18 age and gender matched healthy controls were recruited for this study. The imaging protocol included:
(1) Multi-echo GRE: resolution: 0.5 x 0.5 x 1.25 mm3; TR/TE1: 40/3.77 ms; echo spacing ranging linearly between 4.09 and 4.89 ms, acceleration factor R=2.
(2) MP-FLAIR: TR/TE, 2000/242.8 ms.
The images were registered using FSL tools.8 The registered images were displayed to three image readers (one radiologist and two radiology residents) using the Osirix9 image viewing tool. Readers were instructed to identify the largest 10 lesions for each dataset and identify: (1) lesion size, (2) confluent/non-confluent, lesions (3.1.) presence/absence of vein in a lesion, (3.2.) presence of absence of single/multiple veins in a lesion.
For lesion sizes, the readers first measured the length of the lesion along its longest axis. If this measure was >3-mm, the lesion area was measured.
All subjects with fewer than 3 lesions were removed from analyses. Additionally, all lesions <3-mm in length were removed from the analysis. Based on the resulting set of remaining lesions, three lesion pools were constructed:
(i) large lesions>3-mm (LL), (ii) non-confluent lesions>3-mm (NCLL), (iii) non-confluent lesions>3-mm with single central vein (NCLLSV).
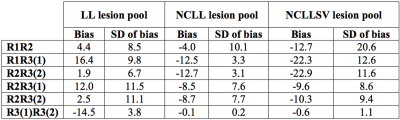
The inter- and intra-reader agreement was evaluated using Bland-Altman analysis. The sensitivity and specificity of CVS as a discriminator of benign versus MS WMLs were analyzed using area under the receiver operating characteristic curve.
Results
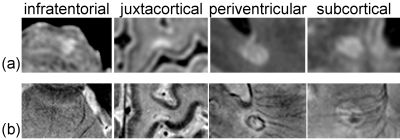
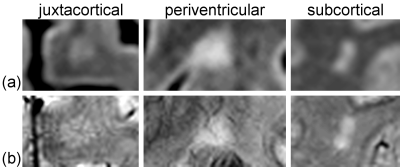
High-field IEV-SWI images were successfully generated for all participants. Figure 1 and 2 show examples (from different subjects) of lesions in different regions of the brain for the RRMS and control group, respectively. Good inter- and intra-reader agreement was observed (Figure 3) supporting the approach of simultaneously visualizing WMLs and the cerebral vessels as a feasible clinical tool. Sensitivity of 94% and specificity of 100% was achieved using a cutoff threshold of 30% for LL pool and 67% for NCLL pool. The removal of the lesions with multiple vessels (the NCLLSV pool) resulted in a sensitivity of only 76% with a cutoff threshold of 66% (Figure 4).Discussion
A channel-by-channel MRI phase combination approach (IEV-SWI) enabled the preservation of phase information while limiting the presence of phase artifacts, allowing for visualization of cerebral vessels of different sizes. The analysis performed as part of this study enabled a new objective derivation of a sensitive and specific radiological definition for CVS: non-confluent lesions >3-mm with one or more vessels. Additional validation of the study should be completed in larger MS cohorts with differing phenotypes to ensure the CVS radiological definition is generalizable.Acknowledgements
No acknowledgement found.References
[1] Tan L, van Schijndel RA, Pouwels PJW, et al. MR Venography of Multiple Sclerosis. Am J Neuroradiol. 2000;21:1039-42.
[2] Grabner G, Dal-Bianco A, Schernthaner M, Vass K, Lassmann H, Trattnig S. Analysis of multiple sclerosis lesions using a fusion of 3.0 T FLAIR and 7.0 T SWI phase: FLAIR SWI. J Magn Reson Imaging. 2011;33(3):543-9.
[3] Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol.
[4] Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12(12):714-22.
[5] Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Investigative Radiology. 2009;44(9):491-4.
[6] Hosseini Z, Liu J, Solovey I, Menon RS, Drangova M. Susceptibility-weighted imaging using inter-echo-variance channel combination for improved contrast at 7 tesla. J Magn Reson Imaging. 2016.
[7] Visser F, Zwanenburg JJ, Hoogduin JM, Luijten PR. High-resolution magnetization-prepared 3D-FLAIR imaging at 7.0 Tesla. Magn Reson Med. 2010;64(1):194-202.
[8] Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782-90.
[9] Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging 2004;17(3):205-216.
Figures
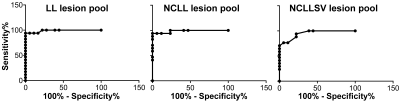
The sensitivity and specificity of CVS as an imaging biomarker for differentiation of WMLs in RRMS from the control group are shown. The pool of lesions >3-mm (LL), AUCLL=0.99, and the pool of non-confluent lesions >3-mm (NCLL), AUCNCLL=0.99, result in higher sensitivity and specificity than non-confluent lesion pool with single vessel (NCLLSV), AUCNCLLSV=0.95.