3298
Age-related neurochemical changes in normal human brain: a proton MR spectroscopy study at 7T1Human Brain Research Center, Kyoto University Graduate School of Medicine, Kyoto, Japan, 2Siemens Healthcare K.K., Japan, Tokyo, Japan, 3Siemens Healthcare, Charlestown, MA, United States
Synopsis
The purpose of this study was to quantify changes of neurochemical concentrations in human brain associated with normal aging with greater sensitivity and accuracy using ultra-high field 7T-MRI. 1H magnetic resonance spectra in the posterior cingulate cortex of 54 healthy adults were measured using a stimulated echo acquisition mode (STEAM) sequence with short echo time, and analysed with LCModel. In addition to the expected result of NAA decrease with aging, both Glutamate and GABA showed significant negative correlations with age. The results may provide significant insights in understanding alterations of human brain accompanying the normal aging.
Introduction
Early detection and prevention of dementia are unmet medical needs around the world. As a premise for investigating the neurodegenerative process, to understand changes in the brain associated with normal aging is indispensable. Proton magnetic resonance spectroscopy (MRS) is a promising method for such a purpose as it can noninvasively assess neurochemical concentrations in human brain in vivo. However, the results of previous studies on age-associated changes of neurochemicals using MRS at 1.5T or 3T are not necessarily consistent1-3, and thus the metabolic changes across life span still remain to be elucidated. This inconsistency of results could be attributable to low sensitivity and specificity which arise from low magnetic fields. Here, we aimed to quantify changes of neurochemical concentrations related to normal aging with high sensitivity and accuracy using ultra-high field 7 T-MRI.Methods
Fifty-four healthy adults (28 females, age;48.8 ± 21.2 (mean ± standard deviation) years, age range; 20–77 years) who had no history of any neurological or psychiatric disorders were enrolled under approval of IRB. Subjects who scored <24 on the Montreal Cognitive Assessment (MoCA) and >10 on the Patient Health Qustionnaire-9 (PHQ-9) were excluded in advance. Two neurologists reviewed all elderly participants to exclude those with incipient neurological disorders. MRS was scanned with a 7T whole-body scanner (Magnetom 7T, Siemens, Erlangen, Germany) using a single-transmit volume coil and a 32-receiver head coil (Nova Medical, MA, USA). A 20-mm cubic volumes-of-interest (VOI) was positioned at the posterior cingulate cortex (PCC) across the mid-sagittal plane on acquired T1-weighted images (Figure 1). Consistent voxel placement among brains was confirmed by the same neuroradiologist for all scans. FASTMAP shimming (prototype) and transmit amplitude adjustment were performed in the MRS voxel. Proton MR spectra were acquired using a stimulated echo acquisition mode (STEAM) sequence (prototype) with short echo time (TR = 8000 ms; TE = 5 ms; mixing time = 45 ms; 32 averages) with water (VAPOR4) and outer volume suppressions. Water unsuppressed spectra of the same MRS voxel were also acquired. All spectra were analyzed using LCModel 6.3-1L (LA Systems, Tokyo, Japan) using the metabolite basis set of alanine (Ala), aspartate (Asp), Cr, GABA, glucose (Glc), glutamine (Gln), glutamate (Glu), glutathione (GSH), GPC, lactate (Lac), mIns, NAA, NAAG, PCr, PCh, scyllo-inositol (sIns), taurine (Tau) and -CrCH2, supplied as a standard option. Eddy current correction and water-scaling for quantification were performed using the water unsuppressed spectra5. Metabolites with mean CRLB value less than 20% were included for further statistical analysis. Associations between age and neurochemical concentrations were analyzed by linear regression. Bonferroni corrections were used to correct for multiple comparisons, and corrected P values below 0.05 were considered statistically significant.Results
A representative MR spectrum, fit curve and residual are shown in Figure 1. Nine neurochemicals were quantified with mean CRLB <20%. Figure 2 shows the results of regression analyses for metabolites and age. Concentrations of NAA, Glu, GABA and tCho showed significant negative correlation with age (P < 0.005) (Figure 3). These correlations remained significant after discarding individual data with CRLB ≧ 20%. Tau and GSH showed weak negative trends (P = 0.01 and 0.02, respectively). Concentrations of tCr, Gln and mIns did not show significant correlation with age.Discussion
In line with some previous reports6, our results showed that NAA, which is thought to be a neuronal marker, decrease with age. Moreover, the results showed that both the main inhibitory and excitatory neurotransmitters in the brain, GABA and Glutamate, significantly decrease with aging. Because regression slopes were steeper in GABA and Glutamate than in NAA, reduction of GABA and Glutamate with aging might reflect not only the results of nonspecific neuronal loss but alterations in regulation of synaptic function. This result is consistent with previous animal studies with MRS7. Although mIns, a possible glial marker, was not correlated significantly across all subjects, there was a tendency for age-related increase in elderly people. It may indicate that mIns increases in non-linear fashion with aging.Conclusion
Comprehensive MRS data of neurochemical alterations related to aging were acquired with 7T ultra-high field scanning. The results may provide a basis for understanding changes in human brain associated with normal aging and will contribute to elucidate disease-specific neurodegenerative process.Acknowledgements
We acknowledge his technical contribution of Dr. Moran R Gerald, Siemens Healthcare Canada.References
1 Reyngoudt H, Claeys T, Vlerick L, Verleden S, Acou M, Deblaere K, De Deene Y, Audenaert K, Goethals I, Achten E (2012) Age-related differences in metabolites in the posterior cingulate cortex and hippocampus of normal ageing brain: a 1H-MRS study. European journal of radiology 81 (3):e223-231.
2. Chang L, Jiang CS, Ernst T (2009) Effects of age and sex on brain glutamate and other metabolites. Magnetic resonance imaging 27 (1):142-145
3. Charlton RA, McIntyre DJ, Howe FA, Morris RG, Markus HS (2007) The relationship between white matter brain metabolites and cognition in normal aging: the GENIE study. Brain research 1164:108-116
4. Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med 1999; 41:649-656.
5. Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, Morrison LA. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn. Reson. Med. 2006; 55(6): 1219–1226.
6. Fayed N, Andres E, Viguera L, Modrego PJ, Garcia-Campayo J (2014) Higher glutamate+glutamine and reduction of N-acetylaspartate in posterior cingulate according to age range in patients with cognitive impairment and/or pain. Academic radiology 21 (9):1211-1217.
7. Duarte JM, Do KQ, Gruetter R (2014) Longitudinal neurochemical modifications in the aging mouse brain measured in vivo by 1H magnetic resonance spectroscopy. Neurobiology of aging 35 (7):1660-1668.
Figures
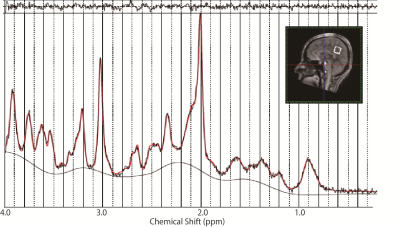
A Representative MR spectrum and the position of a MRS voxel.
A Spectrum was acquired from a 20-mm cubic VOI positioned in the PCC using the short-TE STEAM sequence. A residual is shown on the top of the spectrum.
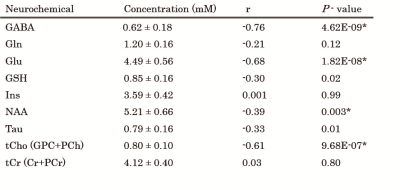
Neurochemical concentrations and P-values of regression analyses with age.
Neurochemicals with mean CRLB <20% were analyzed. *the threshold of significance was set at P=0.0056 (0.05/9) Cr; creaatine, GABA; γ-aminobutyric acid, Gln glutamine, Glu; glutamate, GPC; glycerophosphocholine, GSH; gluthathione, Ins; myo-inositol, NAA; N-acetylaspartate, PCh; phosphocreatine, PCr; phosphocreatine, Tau; taurine, tCho; total Choline, tCr; total creatine
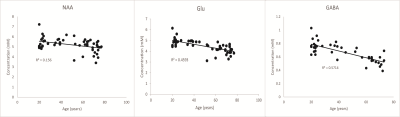
Linear regression plots of metabolite concentrations and age.
NAA, Glu and GABA show significant negative correlation with age (P < 0.005).