3297
Dissecting white-matter fiber pathways of the marmoset brain using ultra-high-resolution diffusion MRI at 7T and 14T1National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, United States, 2The Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, United States, 3National Institute of Mental Health, National Institutes of Health, Bethesda, MD, United States
Synopsis
The common marmoset has received growing interests in neuroscience. The lissencephalic brain of the marmoset grants it experimental advantages for brain mapping and stimulation, but also brings challenges for dissecting white matter tracts, which is poorly depicted in existing marmoset brain atlases. Here, we collected ultra-high-resolution diffusion MRI datasets at 7T and 14T and performed probabilistic tractography and manual segmentation. The marmoset brain demonstrates a complex multi-layer white matter structure with many regions consist of multiple different fiber pathways. With the ultra-high-resolution data, we will provide the first comprehensive map (atlas) for the white matter of the marmoset brain.
INTRODUCTION
The common marmoset, a New-World monkey, has received growing interests in neuroscience. The lissencephalic (smooth) brain of the marmoset grants it experimental advantages over the macaque for brain mapping, recording, and stimulation 1. However, these advantages also come with possible risks that the marmoset brain may be too “simple” (at least looks like simple) to model the complex anatomy and functions of the human brain. These risks can be evaluated via comparative studies and brain atlases. However, most of the marmoset brain atlases available nowadays are focused mainly on the gray matter 2-5, while the white matter structure is poorly depicted. In this study, we aimed to construct a comprehensive white matter atlas of the marmoset brain by using ultra-high-resolution diffusion MRI at 7T and 14T.METHODS
To fully depict the fiber pathways of the marmoset brain, we collected four different diffusion MRI datasets at 7T and 14T with 9 brain samples in total. These unique datasets were the best marmoset diffusion MRI data reported so far, in terms of spatial resolution, b-values and number of directions.
1. Dataset 1 was focused on the number of shells and high b-values (one adult male brain scanned at 7T with a linear transmit-receive RF coil; 150um isotropic; three shells with b=2400, 4800, and 7200 s/mm2; 6 b0 and 126 DWIs per shell; 396 DWIs in total; a full brain coverage).
2. Dataset 2 was focused on the validation with brain samples from different genders and ages (four brains scanned at 7T with a linear transmit-receive RF coil; 150um isotropic; a single-shell with b = 4800 s/mm2; 6 b0 and 126 DWIs; a full brain coverage).
3. Dataset 3 was focused on ultra-high-resolution (one adult male brain at 7T with a newly-designed, customized and high-sensitivity transmit-receive RF coil; 100 um isotropic; two shells with b=2400 and 4800 s/mm2; a full brain coverage).
4. Dataset 4 was focused on ultra-high-resolution (one adult male brain at 14T with a linear transmit-receive RF coil; 80 um isotropic; two shells with b=2400 and 4800 s/mm2. Due to the limited size of the coil at 14T, the frontal pole and occipital pole were not fully covered).
After data preprocessing, we performed probabilistic tractography by Mrtrix3 and we did manual segmentation on the marmoset data.
RESULTS AND DISCUSSION
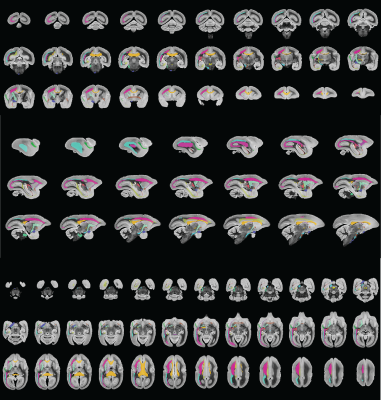
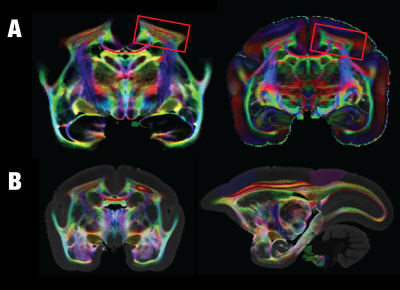
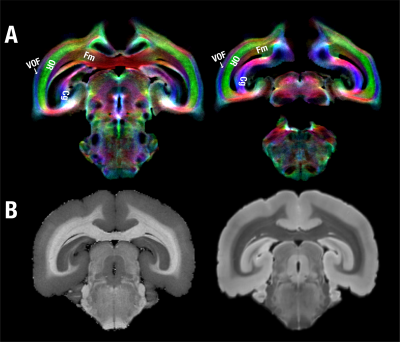
We have, hitherto, identified and segmented 28 white matter structures in the marmoset brain based on probabilistic tractography and manual segmentation (Fig. 1). Although almost lissencephalic, the marmoset brain demonstrates a complex white matter structure. Many white matter regions consist of multiple different fiber pathways. For example, the corona radiata shows at least three-layer structures where fibers of each layer go in different directions. The middle layer goes in an anterior-posterior direction that connects the dorsolateral prefrontal cortex with the posterior parietal cortex and is most likely homologous to the superior longitudinal fasciculus of humans (Fig. 2). In addition to the tracts that connect distant cortices, multi-layer structures can be also observed locally such as in the visual cortex (Fig. 3). Besides the well-defined optic radiation and the forceps major, the vertical occipital fasciculus that directly connects the dorsal and ventral visual systems can also be clearly identified in the marmoset brain, which was a controversial tract that was resolved only recently in humans and macaques 6-7. Due to the almost-lissencephalic brain, the white matter of the marmoset brain appears to be “simple”, but in fact, different fiber tracts are squeezed in complex multi-layer structures. However, these complex structures are hard to be dissected or identified without high-resolution color-coded fiber-orientation images and have not been fully depicted in any previously-published histology or MRI-based atlas. With the ultra-high-resolution data, we will provide the first comprehensive map (atlas) for the white matter of the marmoset brain and will integrate it into the NIH Marmoset Brain Atlas 8.Acknowledgements
We thank Xianfeng (Lisa) Zhang, and Dr. Sang-Ho Choi for their support in animal and brain sample preparation. Also, many thanks go to the High-Performance Computing staff for their support in NIH-HPC computing clusters. This research was supported by the Intramural Research Program of the NIH, NINDS (Alan P. Koretsky, Scientific Director).References
[1] Miller, Cory T., et al. Marmosets: a neuroscientific model of human social behavior. (2016). Neuron 90.2: 219-233.
[2] Paxinos, G., et al. The marmoset brain in stereotaxic coordinates. (2012). Elsevier, AP.
[3] Palazzi, X., et al. The marmoset brain in stereotaxic coordinates. (2008). Springer.
[4] Yuasa, S., et al. Stereotaxic atlas of the marmoset brain: with immunohistochemical architecture and MR images. (2010). National Institute of Neuroscience, National Center of Neurology and Psychiatry, Japan.
[5] Newman, J.D. et al. A combined histological and MRI brain atlas of the common marmoset monkey, Callithrix jacchus. (2009) Brain Res Rev 62, 1-18.
[6] Yeatman, J D., et al. The vertical occipital fasciculus: a century of controversy resolved by in vivo measurements. (2014) Proceedings of the National Academy of Sciences 111.48: E5214-E5223.
[7] Takemura, H., et al. Occipital white matter tracts in human and macaque. (2017) Cerebral Cortex 27.6: 3346-3359.
[8] Liu, C., et al, A digital 3D atlas of the marmoset brain based on multi-modal MRI, Under-Review
Figures