3296
Imaging the perivascular spaces and lenticulostriate arteries at 3T and 7T to provide insight into the pathophysiology of neurodegenerative and neurovascular diseasesGiuseppe Barisano1, Samantha J. Ma2, Danny JJ. Wang2, Yonggang Shi2, Arthur Toga2, and Meng Law1,2
1Radiology, University of Southern California, Los Angeles, CA, United States, 2Stevens Institute of Neuroimaging and Informatics, University of Southern California, Los Angeles, CA, United States
Synopsis
The CSF microcirculation in the brain is not well understood and the role of the perivascular spaces in the clearance of metabolic waste products (including amyloid beta and tau) is still debated. We hypothesize that the increased permeability of the BBB and the resulting passage of blood products into the perivascular spaces may be responsible for the obstruction of CSF-ISF flow. Using a novel T1-weighted 3D Turbo spin-echo with variable flip angles at 3T and 7T, we are able to demonstrate leakage of fibrin from the small lenticulostriate arteries into the perivascular spaces.
Introduction
The CSF microcirculation in the brain is not well understood and while there is controversy surrounding the presence or lack of conventional lymphatics in the brain, high resolution and ultrahigh field MRI (7T and above) is allowing visualization of the perivascular spaces (PVS) and small lenticulostriate arteries (LSA) in humans. This interaction between the LSAs and the PVSs may provide some insight into the passage of metabolic waste products from the blood through the blood brain barrier (BBB) and into the PVSs. The role of the PVSs in this clearance of metabolic wastes is not known1,2. Pre-clinical and some human studies have suggested the drainage of CSF and clearance of metabolic wastes (including amyloid beta and tau) occurring via PVSs and also the intramural peri-arterial drainage (IPAD) pathways3–5. We hypothesize that with aging and brain inflammation, the leakiness and increased permeability of the BBB allows for the passage of metabolic waste products (including fibrin and other blood products) into the PVS. This can obstruct the CSF-ISF (interstitial fluid) flow and PVSs, causing a “fibrin globe” resulting in dilatation of the perivascular spaces6,7.Methods
We studied 15 subjects with vascular risk factors from the Los Angeles Latino Eye Study (LALES) cohort. Young and old healthy subjects were also used as controls. MRI scans were performed on Siemens Prisma 3T and some subjects also on the Siemens Terra 7T MRI scanner at the Center for Image Acquisition, Institute of Neuroimaging and Informatics at University of Southern California. We imaged the lenticulostriate arteries using a T1 high resolution (isotropic 0.5mm) black blood TSE variable flip angle (VFA) sequence and automated 3D vessel reconstruction algorithms8. 3D TSE T2 SPACE sequence was used for the characterization of PVS surrounding the LSAs. 3D TOF MRA and SWI were also performed. We analyzed the relationship between the 3D TOF MRA, T1 VFA TSE black blood, T2 TSE SPACE and the SWI sequence.Results and Discussion
In 4 of the subjects, we found a globe like structure on the T1 VFA BB sequence. These “globes” are felt to represent one of 3 pathologies.
- The first is a fibrin globe, thought to be due to the leakage of fibrin, fibrinogen and other blood products through the BBB into the perivascular spaces. This causes enlargement of the PVS seen in Figure 1 but is negative on the TOF MRA for an aneurysm.
- The second is a true lenticulostriate microaneurysm, so called Charcot Bouchard aneurysms, related to hypertension and other vascular risk factors, which should then be visible on the TOF MRA.
- The third would be a pseudo-aneurysm with blood products in the media of the LSA due to vessel wall lipohyalinosis.
Conclusions
Imaging of the cerebral microvasculature, CSF-ISF microcirculation and perivascular spaces may provide insight into the clearance of metabolic waste products such as amyloid beta in the brain. Using a novel VFA T1 BB sequence at 3T and 7T, we are able to demonstrate leakage of fibrin from the small LSAs into the PVSs. This may be the mechanism leading to obstruction of CSF clearance and dilatation of PVSs, very often described in neurodegenerative diseases such as Alzheimer’s Disease9.Acknowledgements
This study was supported by the US National Institutes of Health grant UH2NS100614 and the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health Award Number P41EB015922. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. #ML partially funded by NIH/NIA P50-AG05142, NIH P01AG052350, NIH P01AD06572.References
- Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111. doi:10.1126/scitranslmed.3003748.
- Smith AJ, Yao X, Dix JA, Jin B-J, Verkman AS. Test of the “glymphatic” hypothesis demonstrates diffusive and aquaporin-4- independent solute transport in rodent brain parenchyma.
- Maclullich AMJ, Wardlaw JM, Ferguson KJ, Starr JM, Seckl JR, Deary IJ. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J Neurol Neurosurg Psychiatry. 2004;75:1519-1523. doi:10.1136/jnnp.2003.030858.
- Patankar TF, Mitra D, Varma A, Snowden J, Neary D, Jackson A. Dilatation of the Virchow-Robin space is a sensitive indicator of cerebral microvascular disease: study in elderly patients with dementia. AJNR Am J Neuroradiol. 2005;26(6):1512-1520.
- Riba-Llena I, Nafría C, Mundet X, et al. Assessment of enlarged perivascular spaces and their relation to target organ damage and mild cognitive impairment in patients with hypertension. Eur J Neurol. 2016;23(6):1044-1050. doi:10.1111/ene.12979.
- Fisher CM. Cerebral Miliary Aneurysms in Hypertension. Am J Pathol. 1972;66(2):313-330.
- Montagne A, Barnes SR, Sweeney MD, et al. Blood-Brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85(2):296-302. doi:10.1016/j.neuron.2014.12.032.
- Ma SJ, Gahm JK, Yan L, et al. Evaluation of Whole Brain and ZOOMit T1-weighted Turbo-Spin Echo (TSE) for Visualization of Human Lenticulostriate Arteries at 3.0 T: A Preliminary Study. Abstract Submitted for Presentation at the Joint Annual Meeting ISMRM-ESMRMB, June 16-21 2018 in Paris, France.
- Tarasoff-Conway JM, Carare RO, Osorio RS, et al. Clearance systems in the brain—implications for Alzheimer disease. Nat Rev Neurol. 2015;11:457-470. doi:10.1038/nrneurol.2015.119.
Figures
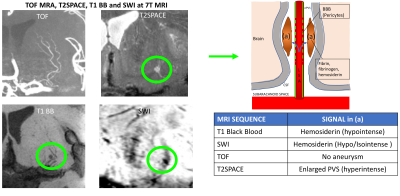
Figure 1: 7T MRI TOF MRA
demonstrating the LSAs originating from the M1 segment of the middle cerebral
artery, no aneurysm is seen. 3D TSE T2 SPACE demonstrates enlarged PVS
associated with the LSA. 3D VFA T1 BB demonstrates a “fibrin globe” originating
from the LSA causing dilatation of the PVS. SWI shows some fibrin and blood
products associated with the PVS and LSA. The diagram shows the mechanism
behind the formation of these “fibrin globes” described in the pathology
literature6 but seen for the first time with MRI.