3285
Longitudinal Evaluation of Post-Surgical Connectivity Changes of the Default Mode Network in Operated Glioma Patients1Center for Mind/Brain Sciences-University of Trento, Trento, Italy, 2Department of Neurosciences, Division of Neurosurgery, “S. Chiara” Hospital, Trento APSS, Trento, Italy, 3Department of Radiology, “S. Chiara” Hospital, Trento APSS, Trento, Italy
Synopsis
In this study we evaluated the post-surgical changes in functional connectivity of the default mode network (DMN) in 6 operated glioma patients and assessed their relationship with pre-operative brain tumor size, a factor that pre-operatively has been negatively associated to cognitive performance. We found in all but one patient an increase in connectivity (average Z-score) of the DMN following brain tumor surgery. These changes were not associated with brain tumor volume, thus indicating that mechanisms other than reduction of mass effect may drive the post-surgical reorganization of the DMN.
Introduction
Intrinsic brain activity (i.e. not in relationship to a specific task) is organized in multiple networks including the default mode network (DMN), whose activation is associated to multiple cognitive functions1. Several studies have reported a decrease of functional connectivity (FC) of the DMN prior to surgery in patients with gliomas in comparison with age-matched healthy subjects, regardless of tumor localization and grade2,3. Therefore in the last few years a vision of glioma not only as a localized disease but also as a pathology that can impact global brain functioning has emerged4. Awake surgery of brain tumor improves the extent of tumor resection with the added benefit of excellent long-term functional outcomes5. However, to the best of our knowledge, no studies have evaluated the longitudinal changes in FC of the DMN following awake surgery and assessed its relationship with clinical variables. In this study we aimed to investigate whether a post-surgical recovery of DMN connectivity could be attributed to the reduction of tumor volume, a factor that pre-operatively has been negatively associated to cognitive performance6.Methods
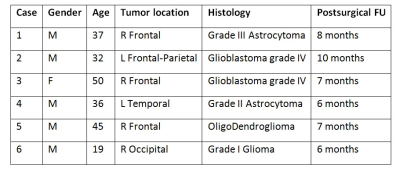
Participants: 6 patients with histopathologically diagnosed gliomas were included in this study approved by the local Ethical Committee. Table 1 reports for each patient demographic and clinical information.
Image acquisition: Each patient underwent an MRI protocol before and after (6-10 months, Table 1) undergoing awake surgery for tumor removal. A 3D T1-weighted gradient echo sequence (axial acquisition, TR/TI/TE=10.64/450/4.23 ms, FA=12°, square FOV=256 mm, voxel size=1x1x1 mm3, ASSET acceleration factor=2) and a 2D T2-weighted FLAIR sequence (axial acquisition, TR/TI/TE=11000/2800/122 ms, square FOV=256 mm, voxel size=1x1x5 mm3, ASSET acceleration factor=2) were acquired for anatomical imaging. A 2D T2*-weighted gradient echo planar imaging (EPI) sequence was acquired for rs-fMRI (axial acquisition, ascending interleaved slice order, TR/TE=2600/45 ms, FA=87°, square FOV=256 mm, voxel size=4x4x4 mm3, slice gap=0.8mm, fat saturation, ASSET acceleration factor=2, 275 volumes).
Image preprocessing: For rs-fMRI images (SPM12 and in house matlab code) the first 4 EPI volumes were removed to allow the signal to reach steady state magnetization, followed by slice timing and head motion correction, median (r=2) filtering, de-trending with a fourth order polynomial and low pass filtering with second-order Butterworth filter (f<0.1 Hz). Head motion shift and rotation parameters and the average time series of white matter (WM) and cerebrospinal fluid (CSF) tissue masks, obtained from the segmentation and co-registration of the T1-weighted images to the rs-fMRI data, were temporally filtered as above and regressed out. Volume outliers were inspected and removed using the ArtRepair software. Finally before FC analysis rs-fMRI data was spatially smoothed using an 8 mm Full Width Half Maximum Gaussian filter and normalized to MNI space using the transformation matrix obtained from the segmentation module. For each patient individually the two rs-fMRI runs were concatenated and then decomposed into 10 independent components7 using the multi-session temporal concatenation procedure in FSL-MELODIC. Each component was thresholded at z-scores > 2.3, P < 0.01. The component with the highest number of voxels in common with a DMN template8 was chosen as representative of the DMN. Dual-regression was then used to derive for each patients the single session DMN. Single-session DMN volume maps were thresholded at z > 2.3, P < 0.01. Tumor volume was derived as the volume of a manually drawn ROIs on the pre-operative T2 FLAIR images that included tumor and surrounding edema (regions of hyperintense signal).
Image post-processing: For each patient we calculated and compared across the two sessions the average Z-score of the DMN using a Wilcoxon-Signed rank test. In order to assess whether the changes in connectivity were associated with brain tumor volume, we calculated the Spearman’s correlation coefficient between brain tumor volume and the difference between post and pre-surgical DMN average z-score (ΔZ).
Results
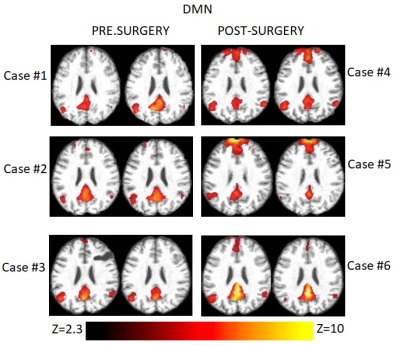
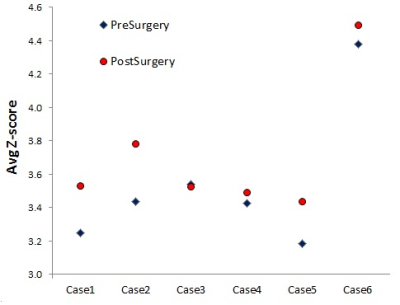
Single-session DMN maps were successfully obtained using dual regression for each patient (Figure 1) The average z-score increased in all but one patient (Figure 2) with trend significance level (n=6, Z=+19, p=0.06). There was no significant correlation between ΔZ and pre-surgical brain tumor volume (ρ=-0.03, p=0.95).Discussion and Conclusions
The results of this study show an increase in FC of the DMN, following awake surgery of gliomas, not associated to brain tumor volume. These findings suggest that mechanisms other than reduction of mass effect may drive the post-surgical reorganization of the DMN. Ongoing work is assessing the relationship between the DMN FC and neurocognitive status changes at multiple longitudinal evaluations.Acknowledgements
This work has been funded by FONDAZIONE CASSA DI RISPARMIO DI TRENTO E ROVERETOReferences
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 2008; 1124:1–38
- Esposito R, Mattei PA, Briganti C et al. Modifications of default-mode network connectivity in patients with cerebral glioma. PLoS One. 2012;7(7):e40231
- Maesawa S, Bagarinao E, Fujii M et al. Evaluation of resting state networks in patients with gliomas: connectivity changes in the unaffected side and its relation to cognitive function. PLoS One. 2015 Feb 6;10(2):e0118072
- Derks J, Dirkson AR, de Witt Hamer PC et al. Connectomic profile and clinical phenotype in newly diagnosed glioma patients. Neuroimage Clin. 2017 Jan 16;14:87-96
- Hervey-Jumper SL, Berger MS. Technical nuances of awake brain tumor surgery and the role of maximum safe resection. J Neurosurg Sci. 2015 Dec;59(4):351-60
- Dalla Bona M, Sarubbo S, Merler S et al. Impact of mass effect, tumor location, age, and surgery on the cognitive outcome of patients with high-grade gliomas: a longitudinal study. Neuro-Oncology Practice Jan 2017, pp. npw030
- Jovicich J, Minati L, Marizzoni M et al. Longitudinal reproducibility of default-mode network connectivity in healthy elderly participants: A multicentric resting-state fMRI study. Neuroimage. 2016 Jan 1;124(Pt A):442-454.
- Yeo BT, Krienen FM, Sepulcre J. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011 Sep;106(3):1125-65.
Figures