3282
Diffusion MR Imaging of Structural Connectivity and Therapeutic Potential of Deep Brain Stimulation in Alzheimer’s Disease Model1Biomedical Engineering, National Yang Ming University, Taipei, Taiwan, 2The Ph.D. Program for Neural Regenerative Medicine, Taipei Medical Univeristy, Taipei, Taiwan
Synopsis
Alzheimer’s disease (AD) is one of the major causes of death that currently cannot be reversed or slowed. Fornix, a major output tract of the hippocampus, has been shown to be a promising target for DBS therapy in AD patients. In this study, triple-transgenic Alzheimer’s mice were used to investigate the changes of white matter integrity and the cognitive functions after the DBS-fornix therapy. We found improvement of the cognition and increased white matter integrity after that DBS-fornix therapy in AD mice. It suggested that the DBS-fornix therapy may be a potential therapeutic intervention of AD.
Introduction
Alzheimer’s disease (AD) is the most common type of dementia with main characteristics including beta amyloid (Aβ) plaques and neurofibrillary tau tangles1 in the brain. Brain circuits have been shown to be greatly involved in AD 2 including the Papez circuit and the limbic circuit. The Papez circuit involves in memory and emotions; including the ability to recall and incite memories 3. The fornix in the limbic circuit as white matter bundles that originates from the hippocampus and then divides into bilateral hemispheres of the brain 4. However, therapeutic intervention of AD is still lack. Deep brain stimulation (DBS) delivers current rectangular pulses to different brain structures as a form of circuitry-based treatment. Since the fornix has input and output pathways with the hippocampus, medial temporal lobe, and nucleus accumbens which relate to memory and emotion, DBS in the fornix may be a candidate for AD therapy. In this study, we applied DBS therapy and MRI scan on the triple-transgenic AD model (3×TgAD) which showed Aβ, tau, and tangle pathologies 5 as well as the phenotypically behavioral aspects found in AD patients. We hypothesized that DBS in the fornix may change the white matter integrity and improve cognitive functions in the 3×TgAD model.Methods
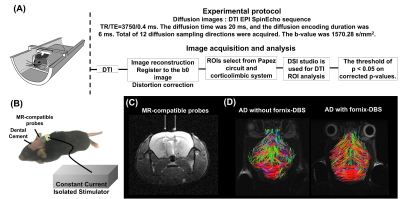
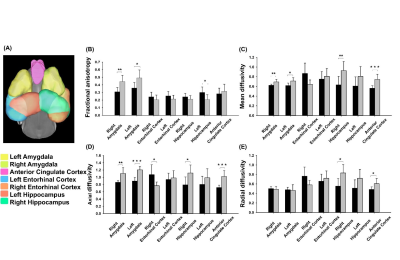
Adult male 3×TgAD mice (weight around 20 g) housed in the animal facility under 12:12-h light/dark cycle with controlling temperature at 22 ± 2°C and then the rats were mated. Novel object recognition (NOR) evaluates the rodents’ ability to recognize a novel object in the environment. It was performed over the course of three days, separated into the habituation day, training day, and testing day. On the habituation day, mice were placed into the test field. Then, they retained memory of the sample objects presented during the training day before to the testing day, when one of the familiar objects is replaced by a novel one 6. A preference index (PI) was calculated using the formula: PI = (n)/(n+f), where n = time with novel object, f = time with familiar object. The experiment design was shown in Fig.1. Two groups, AD with fornix-DBS (DBS-AD, n = 5) and AD without fornix-DBS (shame-AD, n = 5), implanted MR-compatible probes 7,8 in the bilateral fornix (AP: 0.2 mm, ML: 0.2 mm, DV: 2.3 - 2.4 mm). The DBS parameters were set as follows: frequency = 130 Hz, duration = 60 µs, and current = 150 µA. Whole brain images were acquired from a 7 Tesla Bruker MRI (Bruker Biospec 70/30 USR, Ettlingen, Germany). The diffusion tensor images (DTI) were acquired by the DTI EPI Spin-Echo sequence (TR / TE=3750 / 0.4 ms, FOV = 20 × 20 mm2, Matrix: 50 × 50). Region of interests (ROIs) were identified in reference of the C57BI/6j mouse atlas 9 and Allen mouse atlas 10 including the Papez circuit and cortico-limbic system (Fig. 2A). DTI analysis was using DSI Studio (http://dsi-studio.labsolver.org). DTI indices were averaged within each ROIs.Results
Significant higher PI in DBS-AD (57.04%) as compared to the shame-AD (43.11%) (p = 0.005). In the ROI analysis, we found significant differences of DTI indices in the amygdala, hippocampus, anterior cingulate cortex, and entorhinal cortex between the DBS-AD and the shame-AD (Fig. 2B).Discussion
The present study investigated the changes of the cognition and alteration of the white matter in the Papez circuit and corticolimbic system which associated with AD pathology after the DBS-fornix therapy 11,12. Especially, the DTI indices in the hippocampus which involved in memory 13 and spontaneous exploration of novel environments, and in the amygdala which associated with the processing of memory, decision-making, and emotional reactions showed significant differences after the DBS-fornix therapy. The findings imply that the microstructural alteration of the amygdala and hippocampus may attribute to the biological basis of AD, and the DBS-fornix therapy showed therapeutic efficiency of AD.Conclusion
We found improvement of the cognition and increased white matter integrity after that DBS-fornix therapy in AD mice. It suggested that the DBS-fornix therapy may be a potential therapeutic intervention of AD.Acknowledgements
No acknowledgement found.References
1. Sperling R.A., Dickerson B.C., Pihlajamaki M., et al. (2010). Functional alterations in memory networks in early Alzheimer's disease. Neuromolecular Med,12(1):27-43.
2. Jin Y., Huang C., Daianu M., et al. (2016). 3D Tract-Specific Local and Global Analysis of White Matter Integrity in Alzheimer's Disease. Hum Brain Mapp, 38(3): 1191-1207.
3. Duran-Aniotz C., Martínez G., Hetz C. (2014). Memory loss in Alzheimer's disease: are the alterations in the UPR network involved in the cognitive im-pairment? Front Aging Neurosci,6:8.
4. Acosta-Cabronero J., Nestor P.J. (2014). Diffusion tensor imaging in Alzheimer's disease: insights into the limbic-diencephalic network and methodo-logical considerations. Front Aging Neurosci,6:266.
5. Oddo S., Caccamo A., Shepherd J.D., et al. (2003). Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and syn-aptic dysfunction. Neuron, 39(3):409-21.
6. Antunes M., Biala G. (2012). The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process,13(2):93-110.
7. Lai H. Y., Liao L. D., Lin C. T., et al. (2012). Design, simulation and experimental validation of a novel flexible neural probe for deep brain stimulation and multichannel recording. Journal of Neural Engineering, 9(3), 036001.7.
8. Shih Y. Y. I., Yash T. V., Rogers B., et al. (2014). fMRI of deep brain stimulation at the rat ventral posteromedial thalamus. Brain Stimulation, 7(2), 190-193.
9. A.E. Dorr, J.P. Lerch, S. Spring, et al. (2008). High resolution three dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6j mice, NeuroImage, 42(1):60-69.
10. Lein E.S., Hawrylycz M.J., Ao N., et al. (2007) Genome-wide atlas of gene expression in the adult mouse brain, Nature, 445: 168-176.
11. Rowland N.C., Sammartino F., Tomaszczyk J.C., et al. (2016). Deep Brain Stimulation of the Fornix: Engaging Therapeutic Circuits and Networks in Alzheimer Disease. Neurosurgery, 63 Suppl 1:1-5.
12. Acosta-Cabronero J., Nestor P.J. (2014). Diffusion tensor imaging in Alzheimer's disease: insights into the limbic-diencephalic network and methodological considerations. Front Aging Neurosci, 6:266.
13. Ballarini T., Iaccarino L., Magnani G., et al. (2016). Neuropsychiatric subsyndromes and brain metabolic network dysfunctions in early onset Alzheimer's disease. Hum Brain Mapp, 37(12):4234-4247.
Figures