3278
Structural and functional connectivity of the ipsilateral or nondecussating dentato-rubro-thalamic tract1Neurology, Vanderbilt University, Nashville, TN, United States, 2Meharry Medical College, Nashville, TN, United States, 3Biomedical Engineering, Vanderbilt University, Nashville, TN, United States, 4Radiology, Vanderbilt University, Nashville, TN, United States
Synopsis
The dentato-rubro-thalamic tract (DRTT) is a cerebellar efferent pathway important to normal motor function and neurological disease. While the DRTT is described as a decussating (crossing) pathway, the existence of a nondecussating DRTT was recently demonstrated. We compared thalamic connectivity of decussating and nondecussating DRTT using both structural and functional MRI. Probabilistic tractography indicated that the two pathways contact distinct but partially overlapping sets of thalamic nuclei. These results were reinforced by significant correlations with functional connectivity. We conclude that the decussating and nondecussating DRTT exhibit different connectivity patterns, which suggests participation in divergent neural networks.
Purpose.
Our objective was to use high-resolution structural and functional MRI to describe connectivity of the dentato-rubro-thalamic tract (DRTT). The DRTT is a major cerebellar efferent pathway that projects to the thalamus via the red nucleus. It is fundamental for planning and timing of movements. The DRTT is part of the tremor network, and dysfunction in this pathway is implicated in neurological diseases including essential tremor. The existence of a nondecussating (ipsilateral) DRTT (nd-DRTT) was recently demonstrated1. Unlike the classical decussating (crossing) pathway (d-DRTT), the nd-DRTT remains virtually unstudied; therefore, we used diffusion tensor imaging (DTI)-tractography and baseline functional connectivity together with a recently-developed thalamic atlas to compare the pathways in healthy subjects.Methods.
Acquisition. Human Connectome Project data from the WU-Minn consortium were utilized2 (n=92; age=29.3±3.3; sex=54 females). Diffusion-weighted images were acquired at 3.0T (Siemens) using 2D echo-planar readout with 270 directions (TR/TE=5500/89ms; spatial resolution=1.25mm isotropic; t=55min). A multishell diffusion scheme was used with b-values of 1000, 2000, and 3000 sec/mm2. Baseline BOLD scans were acquired (TR/TE=720/33ms; spatial resolution=2mm isotropic; 1200 volumes) and preprocessed using pipelines described in Smith et al.3 T1-weighted images (TR/TE=2400/2.14ms) were acquired to identify the thalamus using FSL-FIRST. Dentate nucleus, superior cerebellar peduncle, and red nucleus were hand-drawn.
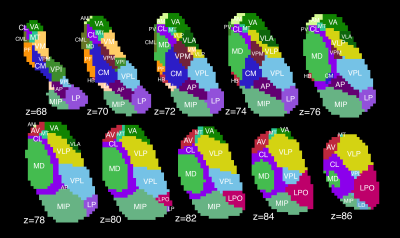
Thalamic segmentation. Thalami were divided into 23 nuclei (Fig. 1) using a novel statistical shape model4 based on prior high-field (7.0T) imaging. Briefly, individual thalami were registered to a shape model based on the Morel atlas5. This method has been cross-validated using a separate cohort of 43 healthy subjects6.
Structural connectivity. Probabilistic tractography was performed using FSL-FDT7, with the dentate nucleus as the seed region, and superior cerebellar peduncle, red nucleus, and thalamus as waypoints. 5000 streamlines were calculated per tract, per subject. Structural connectivity was defined as the percentage of streamlines reaching a given nucleus, after adjusting for size differences between nuclei. “Structural d/nd ratio” was then defined for each nucleus by dividing d-DRTT structural connectivity by nd-DRTT structural connectivity. Thus, d/nd greater than 1 indicated a bias towards the d-DRTT, and d/nd less than 1 indicated nd-DRTT bias.
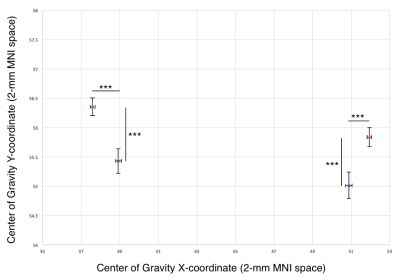
Centers of gravity. To identify the locus of peak structural connectivity, we calculated a weighted center-of-gravity measure for all streamlines reaching the thalamus, producing a single peak Cartesian coordinate for each d-DRTT and nd-DRTT. These coordinates were compared in 2-mm MNI152 space after nonlinear registration.
Functional connectivity. Dentate-to-thalamus functional connectivity was defined using FSL-FEAT. Timecourses from the dentate were taken as inputs in subject-level modeling. Group-level functional connectivity was assessed with a 1-sample t-test using FSL’s randomise function. Thalamic voxels with average functional connectivity parameter estimates greater than 0.3 were included in group-level analysis, and t-statistics reported. We employed this threshold to eliminate weak correlations, similar to the approach in Long et al.8 Z-statistics for each thalamic nucleus were calculated using the Fisher transform. “Functional d/nd ratio” was defined for each of the 23 nuclei by dividing the d-DRTT z-statistic by the nd-DRTT z-statistic. Structural and functional d/nd ratios were compared using a Spearman correlation.
Results.
Structural connectivity. The nd-DRTT structural centers-of-gravity were more posterior in the thalamus than the d-DRTT by an average of 1.8 mm in MNI space (p<0.001), and more medial by 2.42 mm (p<0.001) (Fig. 2).
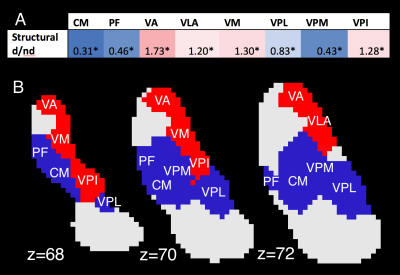
Of the 23 nuclei, 8 received more than 5% of total streamlines from either d-DRTT or nd-DRTT (after size correction). These were: centromedian (CM), parafascicular (PF), ventral-anterior (VA), ventral-lateral-anterior (VLA), ventral-medial (VM), ventral-posterior-lateral (VPL), ventral-posterior-medial (VPM), and ventral-posterior-inferior (VPI). Of these, 4 exhibited statistically significant bias in d/nd ratio toward the d-DRTT (VA, VLA, VM, VPI), and 4 toward the nd-DRTT (CM, PF, VPL, VPM) (p<0.001 for all) (Fig. 3).
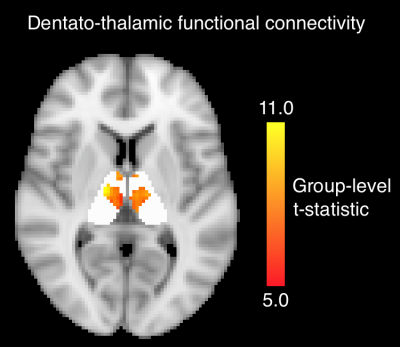
Functional connectivity. Dentate-to-thalamus functional connectivity localized to a distinctive band which ran across the medial aspect of the bilateral thalami, regardless of ipsilateral or contralateral dentate seeding (Fig. 4). There was a significant positive correlation between structural d/nd ratio and functional d/nd ratio across the 23 nuclei (Spearman’s r=0.45, p=0.031).
Discussion.
We found that the newly discovered nondecussating (ipsilateral) DRTT has a characteristic pattern of structural and functional connectivity that differs from its decussating equivalent. As the DRTT is a frequent target for MRI-guided deep-brain stimulation surgery, it is critical to better describe its neuroanatomy. These findings were limited to healthy individuals; future work will focus on neurological disease populations.Conclusion.
The use of both diffusion and baseline BOLD MRI enabled us to confirm that the nondecussating DRTT has unique structural connectivity relative to the conventional decussating pathway, and that its structure is well-matched with its function.Acknowledgements
We are grateful to Yurui Gao and Kurt Schilling for advice regarding tractography, Bennett Landman, Justin Blaber, and Andrew Plassard for assistance with computation, Victoria Morgan for advice, and to the technical staff at Vanderbilt University Institute for Imaging Science.References
1. Meola A, Comert A, Yeh F-C, Sivakanthan S, Fernandez-Miranda JC. The nondecussating pathway of the dentatorubrothalamic tract in humans: human connectome-based tractographic study and microdissection validation. J. Neurosurg. 2016;124:1406–1412.
2. Van Essen DC, Smith SM, Barch DM, Behrens TEJ, Yacoub E, Ugurbil K. The WU-Minn Human Connectome Project: An overview. Neuroimage. 2013;80:62–79.
3. Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, et al. Resting-state fMRI in the Human Connectome Project for the WU-Minn HCP Consortium. Neuroimage. 2013;80:144–168.
4. Liu Y, D’Haese PF, Newton AT, Dawant BM. Thalamic nuclei segmentation in clinical 3T T1-weighted Images using high-resolution 7T shape models. SPIE Med. Imaging. 2015;94150E–94150E.
5. Morel A, Magnin M, Jeanmonod D. Multiarchitectonic and stereotactic atlas f the human thalamus. J. Comp. Neurol. 1997;387:588–630.
6. Chakravorti S, Morgan VL, Trujillo P, Wirz-Gonzalez R, Dawant BM. A Structural Connectivity Approach to Validate a Model-based Technique for the Segmentation of the Pulvinar Complex. Submiss. 2017;
7. Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. In: NeuroImage. 2004. p. S208–S219.
8. Long Z, Duan X, Mantini D, Chen H. Alteration of functional connectivity in autism spectrum disorder: effect of age and anatomical distance. Sci. Rep. 2016;6:26527.
Figures