3257
Morphological Correlates of Objective and Subjective Cognitive Control in Healthy Adults1School of Psychology, Australian Catholic University, Melbourne, Australia, 2School of Psychology, Cardiff University Brain Research Imaging Centre, Cardiff, United Kingdom
Synopsis
There is evidence showing the neural basis of cognitive control utilizing morphological measures of the brain in healthy adults. In the present study, we complement and extend on previous voxel-based morphometry based research by utilizing the more specific brain macrostructure metric of cortical thickness to investigate the differential morphological correlates of objective and subjective cognitive control. Here, we used a rigorous cognitive control test battery implemented on 25 healthy adults. Further research on morphological correlates of objective and subjective cognitive control in healthy populations is necessary to provide baseline data for future clinical populations.
Introduction
Several studies have investigated the neural basis of cognitive control utilizing morphological measures in healthy adults1-4. However, the majority of these studies have utilized the voxel-based morphometry (VBM) approach employing measures such as grey matter density4-6 that are less sensitive than quantitative metrics (e.g., Freesurfer)7. In addition, most studies have focused on “objective” cognitive control, which is an individual’s cognitive ability for a specific cognitive function8. Most studies have neglected “subjective” cognitive control, or one’s self-reporting of their ability to perform cognitive tasks, which captures a different process to “objective” cognitive control8. To overcome these drawbacks, the current study will employ cortical thickness as a specific metric of brain macrostructure to investigate the morphological correlates of objective and subjective cognitive control in healthy adults.Materials and Methods
Participants: 25 healthy adults (Mage=27.68 years; SD=9.36) completed a cognitive test battery including both objective and subjective measures of cognitive control. Objective cognitive control was measured via three subtests of the computerized Psychological Experimental Building Language (PEBL)9 test battery including corsi-blocks (visuospatial memory), digit span (verbal working memory), and vigilance (sustained attention). Subjective cognitive control was self-reported utilizing four subscales of two questionnaires, including the Memory/Attention subscale of the Neurobehavioural Functional Inventory (NFI)10 and the Verbal Memory, Visuospatial Memory, and Attention subscales of the Multiple Ability Self-Report Questionnaire (MASQ)11.
MRI: Anatomical scans were acquired on a Siemens 3T Skyra MRI scanner (Siemens, Erlangen, Germany) with a 32-channel head coil using a three-dimensional magnetisation-prepared rapid gradient-echo (3D-MP RAGE) sequence with the following parameters: TR/TE=2530ms/2.58ms; flip angle=7deg; FOV=220x220mm2; resolution=0.9x0.9x0.9mm3; number of slices=176; TA=6min.
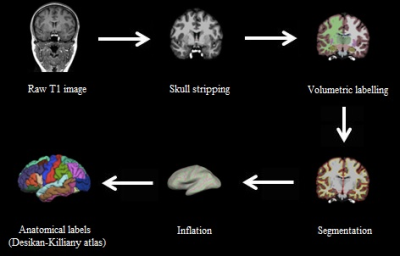
Preprocessing: Freesurfer software version 5.312 was used for cortical reconstruction and volumetric segmentation of the brain surface using a semi-automated approach. An example of the Freesurfer pipeline can be seen in Figure 1. Six regions from the Desikan-Killany atlas13 were selected to define regions of interest (ROIs) comprising the cognitive control network14. Cortical thickness values were extracted for each ROI for left and right hemispheres. The results for each subject were carefully inspected to ensure the accuracy of the skull stripping, segmentation, and cortical surface reconstruction.
Preliminary Correlation Analyses
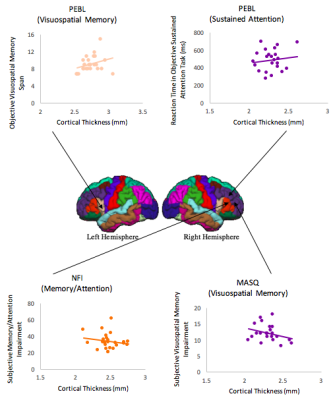
Cortical thickness and objective cognition: There was a significant positive correlation between mean reaction time (RT) in the objective sustained attention test and cortical thickness of the rostral part of the right middle frontal gyrus (r=.51, p=.012, Figure 2). This indicates that increased cortical thickness is associated with worse sustained attention performance. Furthermore, a moderate positive correlation was found between objective visuospatial memory span and cortical thickness of the pars opercularis of the left inferior frontal gyrus (r=.42, p=.035, Figure 2). This relationship indicates that increased cortical thickness is associated with greater visuospatial memory abilities. There were no significant relationships found between participant’s cortical thickness and objective verbal working memory (all p’s>.10).
Cortical thickness and subjective cognition: A significant moderate negative relationship was found between the subjective NFI Memory/Attention subscale and cortical thickness of the pars triangularis of the right inferior frontal gyrus (r=-.41, p= .041, Figure 2). Hence, increased cortical thickness was associated with lower subjective reporting of memory and attention impairments. For the MASQ questionnaire, results revealed a significant moderate negative relationship between visuospatial memory subscale and the rostral part of the right middle frontal gyrus (r= -.58, p= .005, Figure 2, survived FDR correction). In other words, increased cortical thickness was associated with lower subjective self-reported visuospatial memory impairments. Important to note, no other significant correlations were observed between cortical thickness and both Verbal Memory and Attention subscales (all p’s>.10).
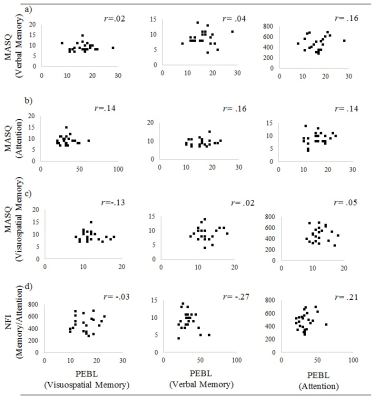
Objective and subjective cognition: Results revealed no significant correlations between participant’s objective and subjective cognitive control. This was across the objective measures three subtests and all subscales of both subjective measures (all p’s>.10). Scatterplots can be seen in Figure 3.
Discussion and Conclusions
Our work complements and extends previous studies that used qualitative VBM-metrics, and used quantitative measures of cortical thickness. It is the first to investigate and provide preliminary evidence for differential morphological correlates of objective and subjective cognitive control in healthy adults. The lack of correlations between objective and subjective cognitive control measures may be due to (a) the study not being sufficiently powered or; (b) the range of subjective cognition being insufficiently large in the healthy population to reveal a correlation. Data acquisition of healthy adults is ongoing due to potential power problems. In conclusion, this study provides important preliminary insights into the differential morphological correlates of subjective and objective cognition, which will serve as a platform for targeted investigations in clinical populations (e.g., Traumatic Brain Injury patients) where these aspects of cognition are differentially impaired.Acknowledgements
This work was facilitated by an ACURF Program grant awarded to Karen Caeyenberghs by the Australian Catholic University (ACU). The authors have no conflict of interest to declare.References
- Antonova E, Kumari V, Morris R, et al. The relationship of structural alterations to cognitive deficits in schizophrenia: A voxel-based morphometry study. Biol Psychol. 2005;58(6):457-467.
- Salthouse TA, Habeck C, Razlighi Q, et al. Breadth and age-dependency of relations between cortical thickness and cognition. Neurobiol Aging. 2015;36(11):3020-3028.
- Schmidt-Wilcke T, Luerding R, Weigand T, et al. Striatal grey matter increase in patients suffering from fibromyalgia–a voxel-based morphometry study. Pain. 2007;132:S109-S116.
- Gautam P, Anstey KJ, Wen W, et al. Cortical gyrification and its relationships with cortical volume, cortical thickness, and cognitive performance in healthy mid-life adults. Behav Brain Res. 2015;287:331-339.
- Bookstein FL. “Voxel-based morphometry” should not be used with imperfectly registered images. Neuroimage. 2001;14(6):1454-1462.
- Crum WR, Griffin LD, Hill DL, et al. Zen and the art of medical image registration: correspondence, homology, and quality. NeuroImage. 2003;20(3):1425-1437.
- Eriksson SH, Free SL, Thom M, et al. Quantitative grey matter histological measures do not correlate with grey matter probability values from in vivo MRI in the temporal lobe. J Neurosci Methods. 2009; 181(1): 111-118
- Miskowiak KW, Petersen JZ, Ott CV, et al. Predictors of the discrepancy between objective and subjective cognition in bipolar disorder: A novel methodology. Acta Psychiat Scand. 2016;134(6):511-521.
- Mueller ST, Piper BJ. The psychology experiment building language (PEBL) and PEBL test battery. J Neurosci Meth. 2014;222:250-259.
- Kreutzer JS, Marwitz JH, Seel R, et al. Validation of a neurobehavioral functioning inventory for adults with traumatic brain injury. Arch Phys Med Rehab. 1996;77(2):116-124.
- Seidenberg M, Haltiner A, Taylor MA, et al. Development and validation of a multiple ability self-report questionnaire. J Clin Exp Neuropsyc. 1994;16(1):93-104.
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. P Natl Acad Sc USA. 2000;97(20):11050-11055.
- Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968-980.
- Metzler-Baddeley C, Caeyenberghs K, Foley S, et al. Task complexity and location specific changes of cortical thickness in executive and salience networks after working memory training. NeuroImage. 2016;130:48-62.
Figures