3246
Brain volumetric and fractional anisotropy differences in mice selected for high and low empathy-like traits1Neuroimaging, King's College London, London, United Kingdom, 2Centre for Behavioural Sciences and Mental Health, Istituto Superiore di Sanità, Rome, Italy, 3Cognitive Neuroscience, Radboud University Medical Centre, Nijmegen, Netherlands
Synopsis
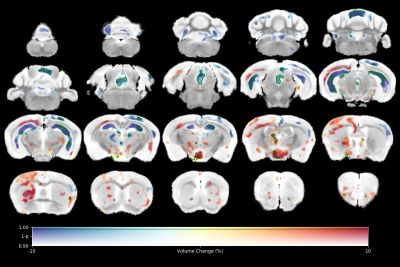
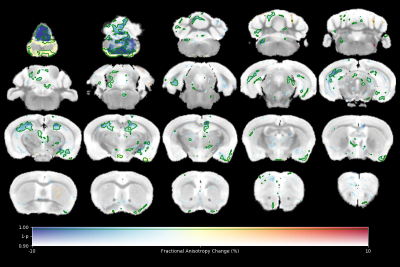
High resolution ex vivo imaging of mice with high and low empathy-like behavior revealed widespread volumetric and fractional anisotropy (FA) changes. Low empathy mice had decreased volumes of the dorsal and ventral hippocampi, periaqueductal grey and the cerebellar cortex, and increased volumes of the olfactory bulb and the hypothalamus compared to high empathy mice. FA was decreased in the low empathy group, specifically in the hippocampus and in the periaqueductal grey. Functional significance can be inferred as these affected brain circuits mediate olfactory cues-based communication of pain, predatory odor fear responses and autonomic stress responses.
Introduction
MRI is increasingly used for investigating the neural circuits underlying phenotypes in animal models of psychiatric traits. Multiparametric imaging can reveal areas of the brain exhibiting changes that are often ‘downstream’ from the original dysfunctional neuro-circuit or lesion. Structural differences persist in the tissue even at post-mortem which enables non-invasive evaluation of brains before further processing for histological or biochemical biomarkers1. Here, we performed high resolution ex vivo imaging of mice that were behaviorally characterized as demonstrating high or low empathy-like behavior, based on individual responses in a social modulation of pain test 2,3. The test involves injecting a low dose of formalin into the paws of experimental mice and measuring their response to pain in the presence of a familiar mouse injected with higher dose of formalin. “High empathy” mice spend more time licking their paw when in visual and olfactory contact with the mouse experiencing a higher degree of discomfort compared to the “low empathy” subgroup. We acquired structural, DTI and relaxometry (T1 and T2) images, and performed voxel-wise comparisons between the two groups of mice.Methods
Male adult Balb/cJ mice were behaviorally tested and separated into high (n=11) and low (n=12) empathy groups. Mice were killed by transcardiac perfusion with saline followed by 4% paraformaldehyde. Their heads were stored in phosphate-buffered saline with 0.5% sodium azide for ca. 5 months. Heads were imaged immersed in perfluoropolyether (Galden, Solway), four at a time4, in a 9.4T Bruker scanner using a 39mm birdcage RF coil (Rapid). High resolution (100 micron isotropic) structural images and quantitative T1 and T2 maps were acquired using a modified DESPOT1 and DESPOT2-FM protocol4 that includes FLASH (TR 48ms, TE 7.695ms, FA 7°,17°,41°), True-FISP (TR 8ms, TE 4ms, FA 15°, 30°,45°) and AFI (TR 20ms, TE 2.7ms, FA 55°) scans. In addition, a diffusion tensor imaging (DTI) scan was acquired (TR 4s, TE 22.6ms, 30 directions at b=1500, 4 at b=0) with 200 micron isotropic resolution from which the maps of fractional anisotropy (FA) and mean diffusivity (MD) were calculated. A group analysis was carried out on all relaxometry maps, DTI maps and the Jacobian determinant images with permutation tests and Threshold-Free Cluster Enhancement (TFCE) using FSL randomize4.Results
The results of tensor-based morphometry analysis revealed changes in several brain areas (Figs 1&3). Changes include decreased volumes of the dorsal and ventral hippocampi, periaqueductal grey (PAG) and the cerebellar cortex; as well as increased volumes of the olfactory bulb and the hypothalamus in the low compared to high empathy mice. Fractional anisotropy (FA), on other hand, was only decreased in the low empathy group, specifically in the hippocampus and in the periaqueductal grey (Figs 2&3). There were no significant differences between groups in MD, T1 or T2. This also suggests that the observed FA and volume changes are unlikely to be caused by underlying partial volume or T1 effects.Discussion
This was an exploratory imaging study of animals segregated by a behavioral phenotype: their social response to pain. There were significant differences in brain volumes and FA in circuits that mediate olfactory cues-based communication of pain, as well as those involved in predatory odor fear responses. Olfactory systems coupled with PAG are known to process complex predator chemosignals, which also involves the hippocampus 5,6. Such responses, signaling via amygdala, activate the hypothalamus-pituitary-axis and the autonomic stress responses. Although we only detected weak unilateral amygdala changes, the area of medial hypothalamus, possibly including the paraventricular nucleus, was significantly affected. Interestingly, there is an accumulation of evidence in both humans and rodents that points toward an under-appreciated involvement of olfactory circuits in stress, resilience and fear7. Moreover, imaging, behavioral and genetic evidence implicates cerebellar dysfunction, specifically reduced volume and FA, in autism spectrum disorders, where it is known, in both humans and experimental animals that low empathy is one of key features8. FA changes are typically associated with white matter abnormalities and it is possible that here lower FA reveals lower (or disorganized) myelination in the low empathy mice. Morphometry results may indicate alterations in the size and number of different cell types, both neurons and glia. Further analysis of brain changes associated with these macroscopic imaging metrics will help to unmask the underlying cellular pathology.Conclusion
High resolution structural imaging revealed subtle differences in regional brain volume and fiber organization in a mouse model of high vs. low empathy-like behavior. Affected brain areas comprise circuits that mediate fear behavior, olfactory processing and autistic-social disorders. These findings provide guidance toward further exploration of the relevant brain areas by histological and genetic means in order to improve our understanding of the neurobiology of social behavior.Acknowledgements
No acknowledgement found.References
1. Lerch et al. (2017) Nat Neurosci 20, 314–326.
2. Langford et al. (2006) Science 312(5782): 1967.
3. Gioiosa et al. (2009) PLoS One 4(1): e4143.
4. Wood et al. 2016. PeerJ 4, e2632.
5. Takahashi (2014) Front in Beh Neurosci. 8:1
6. Smith et al. (2016) Science Advances 2(10)e1600855
7. Chen et al. (2016) Psychiatry Research 245:36
8. Silverman et al. (2010) Nat Rev Neurosci 11(7):490
Figures