3245
Accuracy of Morphometry Measures from MPRAGE data with Prospective Motion Correction Based on an Optical Tracking System1NINDS/NMRF, National Institutes of Health, Bethesda, MD, United States, 2NIMH/DNU, National Institutes of Health, Bethesda, MD, United States, 3NIMH/fMRIF, National Institutes of Health, Bethesda, MD, United States, 4MR Development and Application Center, University Medical Center Freiburg, Freiburg, Germany
Synopsis
Subject motion during MRI results in poor image quality and may cause bias in the morphormetric measures extracted from segmentation algorithms. Prospective motion correction (PMC) techniques can mitigate these effects by tracking brain motion and updating the scan parameters in realtime. Here, we compared the accuracy of cortical thickness and volume extracted from MPRAGE data of non-moving and intentionally moving subjects when using a PMC method based on a Moire phase tracking marker and an optical system. Data show that the PMC method used here can greatly reduce image artifacts and provide more accurate segmentation resuIts during intentional motion.
Introduction
Subject motion has a deleterious effect on high-resolution structural MRI. The image artifacts from motion not only result in poor image quality, but also can bias the anatomical morphormetric measures extracted from segmentation algorithms1. Prospective motion correction (PMC) techniques can mitigate these effects by tracking brain motion and updating the scan parameters accordingly2. Head motion can be monitored either by utilizing MR navigator data or by using external hardware. It has been shown that the reproducibility and accuracy of cortical and subcortical measures can be significantly improved when using navigator-based PMC techniques in the presence of motion3-6. In this study, we compare morphometry measures extracted from MPRAGE data of non-moving and intentionally moving subjects when using a PMC method based on an optical motion tracking system.Methods
MPRAGE data were acquired on healthy adults (n=3) at 3T (Skyra, Siemens Healthcare, Erlangen, Germany) with a 32-channel head coil using PMC with the following scan parameters: TE/TR=2.2/2530ms, IR=1100ms, ESP=6.6ms, FA=7°, iPAT = 2, 1x1x1mm, scan time = 6 min 2 s. PMC was accomplished using a Moire phase tracking marker and an optical system (KinetiCor, HI, USA)7. The marker was affixed to a plastic rod (4 cm) glued to a sports mouth guard (ArmourShield, Under Armour, MD, USA) customized for each subject by molding to fit the upper teeth. The motion parameters generated by the camera were captured by the scanner and used to update gradient amplitudes and frequency and phase of the RF pulses to maintain the scan plane and FOV before acquisition of each k-space line. Subjects were instructed either not to move or perform alternating side-to-side and nodding motions 3 times when cued, yielding about 20 seconds of movement 5 times (once every minute) during the scan. The image data were acquired under 4 different conditions i) No intentional motion and no PMC (reference condition); ii) With intentional motion and with PMC; iii) No intentional motion and with PMC; iv) With intentional motion and no PMC. Data from each condition were acquired twice. Measurements of cortical thickness and volume were extracted using FreeSurfer 5.38 and values for each lobe were calculated by combining the relevant regions. The accuracy of each morphometry measure was estimated for each experimental condition as the normalized percent difference from the reference condition averaged across subjects.Results
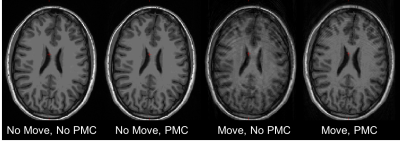
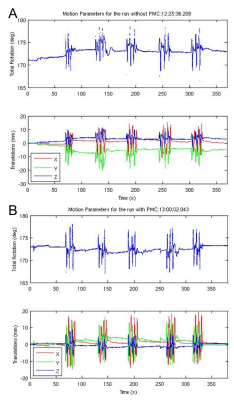
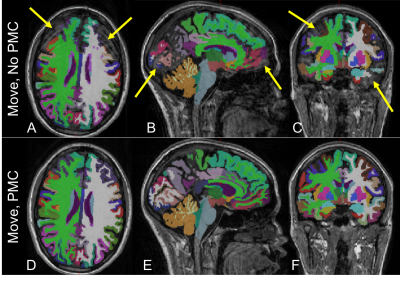
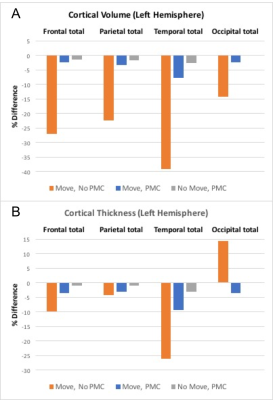
All subjects completed the scans and none reported any discomfort due to the mouth guard. Representative images from one subject are shown in Fig. 1. Images show significant reduction in image artifacts during the intentional motion condition with PMC compared to no PMC condition. In addition, it can be seen that the images acquired in the no-move conditions with and without PMC agree very well. Figure 2 shows the correponding motion parameters of both runs (without and with PMC) when intentional motion was performed. The within-subject motion was similar between the experimental conditions (Figs. 2A and 2B). The translations and rotations during intentional head motion were within +/-10mm and +/-5°, respectively. Figure 3 shows the segmentation results from FreeSurfer for the same subject in Fig. 1. Figure 3 A-C show that the artifacts from motion result in segmentation errors, as indicated by yellow arrows. These gross errors in segmentation are eliminated with PMC, as seen in Fig. 3. D-F. Figure 4 shows the the calculated accuracy of cortical volume and thickness in the left hemisphere. As expected, with subject motion and no PMC, morphometry measures contain errors from 5-39%. When PMC is applied, the data show very good agreement (within ~5%) with the reference condition. Similar results were obtained for the right hemisphere lobes (data not shown).Discussion
The accuracy of morphometry measures extracted from FreeSurfer were significantly affected by subject motion, with a bias towards decreased volume and thickness. In this preliminary study, we observed that prospective motion correction using optical tracking can be used to significantly reduce image artifacts and provide more accurate segmentation resuIts during intentional motion. We believe the type of mouth guard used here (attached to the upper teeth only) will be well tolerated by most subjects. Image quality with PMC may be further improved by using a fat-saturated MPRAGE protocol and rejecting echo trains during the onset of most rapid movements to reduce residual ringing. These refinements will be investigated in future studies with a larger number of subjects.Acknowledgements
No acknowledgement found.References
[1] Reuter M, et. al., ‘Head motion during MRI acquisition reduces gray matter volume and thickness estimates’, NeuroImage 2015;107:107-115.
[2] Maclaren J, et. al., Prospective motion correction in brain imaging: a review. Magn Reson Med 2013;69:621–636.
[3] Brown TT, et al. Prospective motion correction of high-resolution magnetic resonance imaging data in children. Neuroimage. 2010;53:139-45.
[4] Sarlls JE, et. al., Contribution of FOV Updating and Reacquisition to Estimates of Cortical Surface Measures in PROMO MPRAGE., Proc. Intl. Soc. Mag. Reson. Med. 2015;23:2551
[5] Tisdall MD, et. al., Prospective motion correction with volumetric navigators (vNavs) reduces the bias and variance in brain morphometry induced by subject motion. Neuroimage. 2016;127:11-22.
[6] Watanabe K, et. al,. Utility of real-time prospective motion correction (PROMO) on 3D T1-weighted imaging in automated brain structure measurements. Sci Rep. 2016;6:38366.
[7] Maclaren et. al., (2012), Measurement and Correction of Microscopic Head Motion during Magnetic Resonace Imaging of the Brain, PLOS ONE Vol 7(11), e48088.
[8] Fischl, et. al., Whole Brain Segmentation: Automated labeling of Neuroanatomical Structures in the Human Brain, Neuron, (2002);33:341-355.
Figures