3242
Correlation of baseline DSI based metrics with clinical motor outcomes at 6 weeks after acute ischemic stroke1Bioengineering, University of Utah, Salt Lake City, UT, United States, 2Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States, 3Occupational Therapy, University of Utah, Salt Lake City, UT, United States, 4Neurology, University of Utah, Salt Lake City, UT, United States, 5Waisman Center, University of Wisconsin, Madison, WI, United States
Synopsis
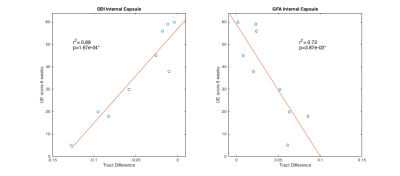
The difference between the baseline ipsilesional and contralesional mean values in the internal capsule from Orientation Dispersion Index and Generalized Fractional Anisotropy correlate strongly with upper extremity clinical outcomes at 6 weeks. These models account for regions of crossing fibers and demonstrate improvements over DTI in using brain microstructure to make clinical judgments.
Motivation and Introduction
After acute ischemic stroke, patients and providers face a dilemma – what to do next? Recommend expensive drugs and therapy? Furthermore, upon what evidence should a decision to begin drugs or therapy be based? The current methods for predicting stroke outcomes, including baseline disability levels, age at stroke onset, infarct volume, and lesion location, all have limitations. In addition to these methods, white matter integrity is considered important to cognitive function and Diffusion Tensor Imaging (DTI) measures such as Fractional Anisotropy (FA), Mean Diffusivity (MD), Radial Diffusivity and others have also been shown to have some predictive value in assessing stroke outcomes, especially when paired with Transcranial Magnetic Stimulation1-3.However, the DTI measures do not account for regions of crossing fibers and therefore do not adequately capture the characteristics of brain microstructure4. To test the hypothesis that accounting for regions of crossing fibers may have clinical importance, we compare the traditional DTI measures of FA and MD to higher order models of brain microstructure, namely Generalized Fractional Anisotropy (GFA) and the 3 parameters of the Neurite Orientation Density and Dispersion Imaging (NODDI) model5. Specifically, we present the beginnings of an improved prognosis pipeline based on location specific metrics from higher order Diffusion Spectrum Imaging (DSI) models.Methods
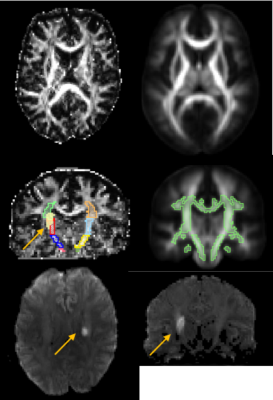
Data acquisition: DSI data with a b-max of 4000 and 203 directions6,7 was acquired in 9 stroke subjects (age 69±8.5 yrs) on a Siemens 3T Verio scanner using a 32 channel head coil ≤12 days post-stroke. A simultaneous multi-slice blipped CAIPI sequence8,9 was used with a slice acceleration factor of three. The scan parameters were TR=3.7 sec, TE=114.2 msec, number of slices=51, slice thickness=2.1mm, total data acquisition time=13 minutes. Data Processing: Figures 1 and 2 explain processing flow10-18. We calculated the coefficient of determination r2 for the mean and median values in corticospinal tract segments with the baseline Upper Extremity Fugl-Meyer score taken 8±2 days post stroke (UEb), and the UE at 6±2weeks post stroke (UE6). Lastly, we calculated r2 for the difference of the mean and median values in the ipsilesional and contralesional tract segments with UEb and UE6.Results
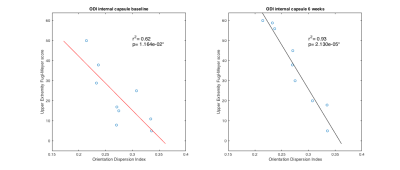
Hemispheric Differences: The largest correlations between scan metrics and UE scores occurred in the difference between the mean value of the ipsilesional and contralesional internal capsule in GFA and ODI maps (Fig 3). Slope with UE6 in the cerebral peduncle was negative for GFA (r2=0.42) and positive for ODI (r2=0.48). For all other areas and parameter maps, r2 was <|0.4|. Ipsilesional and Contralesional Tract Metrics: Values of the mean ODI in the ipsilesional internal capsule show negative slope with UEb (r2=0.62) and UE6 (r2=0.94) (Fig 4). Mean RDI values show positive slope (r2=0.52) in the ipsilesional corona radiata with UEb as well as in the contralesional internal capsule (r2=0.53) and corona radiata (r2=0.66) with UE6. All remaining regions of ODI and RDI and values of MD, FA, CSF, and GFA in ipsilesional and contralesional tract regions versus UEb and UE6 had r2=<|0.4|. The correlation coefficient of both age and infarct volume with UEb and UE6 was <|0.5|. Two-tailed t-test showed significant difference between male and female outcomes (male:n=5; female:n=4), however the oldest subject with the most severe stroke was male.Discussion and Conclusion
The strong correlation between hemispheric differences in the internal capsule in the GFA and ODI models and clinical outcomes is an exciting step towards better ischemic motor stroke prognosis (Fig 3). Our results support others’ findings that tract specific analysis provides superior information to infarct volume, and goes beyond previous work by analyzing more than just DTI measures, the stroke ROI itself, or overlap of the stroke ROI with tracts1-4,19,20. Granziera et al. reported correlation between 6 month outcomes and GFA values in the contralesional hemisphere in the acute phase after stroke onset19. While our data do not show the same strong correlation for GFA, the contralesional RDI values do support the Granziera hypothesis. In addition to contralesional changes, we show that the ODI value in the ipsilesional tract is strongly correlated with stroke outcomes (Fig 4). One limitation of our study is we did not differentiate between fiber origins in premotor cortex, primary cortex, or supplementary motor regions, and this may explain the lack of strong correlation between the corona radiata and stroke outcomes as well as the differences between our study and Granziera. In conclusion, DSI based metrics better correlate with clinical upper extremity outcome than DTI based metrics and could eventually be used to recommend therapy or to personalize therapy.Acknowledgements
This work is supported by R01NS083761.References
[1] C. Doughty, J. Wang, W. Feng, D. Hackney, E. Pani, and G. Schlaug, “Detection and Predictive Value of Fractional Anisotropy Changes of the Corticospinal Tract in the Acute Phase of a Stroke,” Stroke, vol. 47, no. 6, pp. 1520–1526, May 2016. [2] J. Puig, S. Pedraza, G. Blasco, J. Daunis-i-Estadella, F. Prados, S. Remollo, A. Prats-Galino, G. Soria, I. Boada, M. Castellanos and J. Serena, "Acute Damage to the Posterior Limb of the Internal Capsule on Diffusion Tensor Tractography as an Early Imaging Predictor of Motor Outcome after Stroke", American Journal of Neuroradiology, vol. 32, no. 5, pp. 857-863, 2011. [3] C. Stinear, P. Barber, M. Petoe, S. Anwar and W. Byblow, "The PREP algorithm predicts potential for upper limb recovery after stroke", Brain, vol. 135, no. 8, pp. 2527-2535, 2012. [4] G. Adluru, Y. Gur, J.S. Anderson, L.G. Richards, N. Adluru, E.V.R. DiBella, Assessment of white matter microstructure in stroke patients using NODDI, in: Engineering in Medicine and Biology Society (EMBC), 2014 36th Annual International Conference of the IEEE, 2014, pp. 742-745. [5] H. Zhang, T. Schneider, C. Wheeler-Kingshott and D. Alexander, "NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain", NeuroImage, vol. 61, no. 4, pp. 1000-1016, 2012. [6] S. Moeller, E. Yacoub, C.A. Olman, E. Auerbach, J. Strupp, N. Harel, K. Ugurbil, Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI, Magnetic Resonance in Medicine, 63 (2010) 1144-1153. [7] K. Setsompop, B.A. Gagoski, J.R. Polimeni, T. Witzel, V.J. Wedeen, L.L. Wald, Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty, Magnetic Resonance in Medicine, 67 (2012) 1210-1224. [8] B. Bilgic, I. Chatnuntawech, A.P. Fan, K. Setsompop, S.F. Cauley, L.L. Wald, E. Adalsteinsson, Fast image reconstruction with L2-regularization, Journal of Magnetic Resonance Imaging, 40 (2014) 181-191. [9] L.-W. Kuo, J.-H. Chen, V.J. Wedeen, W.-Y.I. Tseng, Optimization of diffusion spectrum imaging and q-ball imaging on clinical MRI system, NeuroImage, 41 (2008) 7-18. [13]S.M. Smith. Fast robust automated brain extraction. Human Brain Mapping, 17(3):143-155, November 2002. [10] Veraart J et al. (2016)] "Diffusion MRI noise mapping using random matrix theory", Magnetic Resonance in Medicine, in press, DOI: 10.1002/mrm.26059 [11] University Medical Center Freiburg, Dering software license. https://www.uniklinik-freiburg.de/roentgen-en.html [12] Jesper L. R. Andersson and Stamatios N. Sotiropoulos. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage, 125:1063-1078, 2016. [13] Jesper L. R. Andersson, Mark S. Graham, Eniko Zsoldos and Stamatios N. Sotiropoulos. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. NeuroImage, 141:556-572, 2016. [14] P. A. Cook, Y. Bai, S. Nedjati-Gilani, K. K. Seunarine, M. G. Hall, G. J. Parker, D. C. Alexander, Camino: Open-Source Diffusion-MRI Reconstruction and Processing, 14th Scientific Meeting of the International Society for Magnetic Resonance in Medicine, Seattle, WA, USA, p. 2759, May 2006. [15] Garyfallidis E, Brett M, Amirbekian B, Rokem A, van der Walt S, Descoteaux M, Nimmo-Smith I and Dipy Contributors (2014). Dipy, a library for the analysis of diffusion MRI data. Frontiers in Neuroinformatics, vol.8, no.8. [16] Yeh F-C, Wedeen VJ, Tseng WY (2010), “Generalized Q-Sampling Imaging”, IEEE Trans. Med. Imaging. [17] Johns Hopkins University DTI-based white-matter atlas. https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases; [18] Advance Normalization Tools (ANTs). https://sourceforge.net/projects/advants/ [19] C. Granziera, A. Daducci, D. Meskaldji, A. Roche, P. Maeder, P. Michel, N. Hadjikhani, A. Sorensen, R. Frackowiak, J. Thiran, R. Meuli and G. Krueger, "A new early and automated MRI-based predictor of motor improvement after stroke", Neurology, vol. 79, no. 1, pp. 39-46, 2012. [20] J. D. Schaechter, Z. P. Fricker, K. L. Perdue, K. G. Helmer, M. G. Vangel, D. N. Greve, and N. Makris, “Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients,” Human Brain Mapping, vol. 30, no. 11, pp. 3461–3474, 2009.
Figures
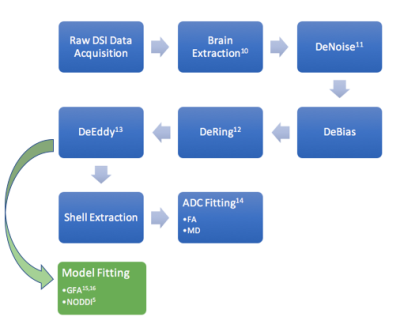
Figure 1-The preprocessing steps to remove artifacts and
noise are the same for the DSI and DTI pipelines until after the DeEddy step.
From there the data is split into shells for the DTI model fitting or used
directly for GFA and NODDI model fitting. The NODDI model consists of
three parameter maps: Cerebrospinal Fluid (CSF), Restricted Diffusion Index
(RDI) and Orientation Dispersion Index (ODI) which are all scaled between 0 and
1.