3240
Optimal tissue preparation for ex vivo preclinical imaging1Department of Neuroimaging, IOPPN, King's College London, London, United Kingdom, 2Natbrainlab, Department of Forensic and Neurodevelopmental Sciences, IOPPN, King's College London, London, United Kingdom, 3Department of Basic and Clinical Neuroscience, IOPPN, King's College London, London, United Kingdom, 4MRC Centre for Neurodevelopmental Disorders, King's College London, London, United Kingdom
Synopsis
Ex vivo imaging is beneficial for studying rodent brain microstructure in healthy and pathological tissue at high resolution. There are challenges however associated with changes in tissue properties resulting from fixation. We present a tissue preparation protocol optimised for diffusion MRI in the rat brain by varying fixative concentration, gadolinium concentration and rehydration time. By altering T1 and T2 relaxivity, we show how these factors can be combined to maximise SNR efficiency. Improving SNR efficiency in ex vivo diffusion MRI will allow higher spatial and angular resolution for studying tissue microstructure in the rodent brain.
Introduction
Preclinical ex vivo magnetic resonance imaging (MRI) is advantageous for imaging the rodent brain at microscopic scale through combination of high field strengths (>7T) and long imaging times. Diffusion MRI in particular offers unique opportunities to study microstructure and three-dimensional organization of white matter fibers in the rodent brain at high resolution, in normal animals and disease relevant models. However due to changes in tissue properties after perfusion fixation1,2 ex vivo MRI is not without challenges. The reduced T2 in fixed tissue and long TE necessary for diffusion contrast limits the signal to noise ratio (SNR). Here, we present a new tissue fixation protocol optimized for diffusion MRI. T1/T2 can be optimized by varying fixative concentration3-5 in perfusion, rehydration time after fixation6,2,7 and amount of gadolinium contrast agent in ‘active fixation’8,9. We demonstrate how these factors can be combined for an optimal MRI tissue preparation procedure, through maximization of SNR efficiency.Methods
Animal preparation
Adult male rats (Sprague Dawley, n=12) were euthanized with pentobarbital (60 mg/kg i.p.) and transcardially perfused with ice-cold 0.9% saline followed by either 2% or 4% paraformaldehyde (PFA, ‘Parafix’, Pioneer Research Chemicals, UK) containing Gd-DTPA (Magnevist) in a concentration range of 0-50mM. The heads were removed and stored in the fixation solution for 4 days, then transferred to phosphate buffered saline (PBS) with either 0 or 1mM Gd-DTPA, at +4°C. Rats (n=2) were scanned at intervals between 0-35 days of rehydration, with the remainder (n=10) at 35 days. Heads were moved to room temperature 4 hours before scanning.
MRI acquisition
MR imaging was performed at the KCL BRAIN Centre (https://brain-imaging.org), on a Bruker 9.4T scanner with Rapid 39mm volume coil. For T1 mapping, a rapid acquisition with relaxation enhancement (RARE) sequence was used with 6 TRs from 200-5500ms, TE=7ms. For T2 mapping, a multi-slice multi-echo sequence (MSME) was used with 30 TEs from 8-240ms, TR=2000ms. Both acquisitions had in-plane resolution 0.23x0.23mm and slice thickness 1mm.
Analysis T1 and T2 maps were obtained using Bruker Image Sequence Analysis tools. Mean and standard deviation of T1 and T2 were measured in a 10-voxel region of interest placed at the midpoint of the corpus callosum. Curves relating T1 and T2 to [Gd-DTPA] were fitted in Matlab as2
$$\frac{1}{\text{T}_{i\text{_observed}}}=\frac{1}{\text{T}_{i\text{_tissue}}}+\frac{\text{[Gd-DTPA]}}{\text{T}_{i\text{_Gd-DTPA}}}$$
These values were then used to calculate SNR efficiency to show the best choice of [Gd-DTPA] and TR, for different values of TE, using2:
$$\text{SNR}_{\text{efficiency}}\propto \frac{1}{\sqrt{\text{TR}}}e^{\frac{-\text{TE}}{\text{T2}}}[1-e^{\frac{-\text{TR}}{\text{T1}}}(2e^{\frac{-\text{TE}}{2\text{T1}}}-1)]$$
Results and Discussion
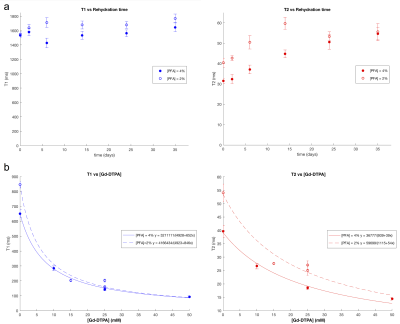
Figure 1a shows how T1 and T2 change with time spent soaking in PBS after fixation. T1 is relatively stable between 1400-1600ms from 0-35 days post fixation, whereas T2 increases steadily from 30 to 50ms in 4% PFA-fixed samples, and 2% PFA-fixed samples from 40 to 50ms, plateauing after approximately 4 weeks. The gain in T2 resulting from the 2% PFA is also evident in Figure 1b, and was reproduced in two additional rats not shown here, prepared without gadolinium, scanned only at 35 days. Longer T2 is advantageous for diffusion MRI because the TE needs to be longer than normal structural imaging to allow adequate diffusion time and diffusion contrast without compromising signal due to T2 relaxation.
Figure 1b shows the effect of adding gadolinium contrast agent to the perfusion and rehydration solutions. T1 decreases more rapidly with respect to [Gd-DTPA] than T2, for rats prepared both with 2 and 4% PFA. Reducing T1 with gadolinium can improve signal intensity, spatial and/or angular resolution in a given acquisition time. However, gadolinium also reduces T2, to a lesser extent, so this must be taken into account when choosing the optimal concentration.
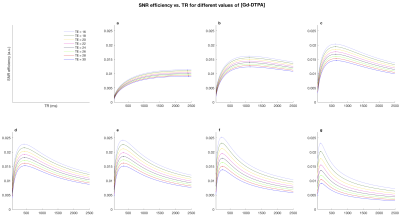
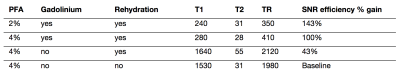
The effect of gadolinium on SNR efficiency is shown in Figure 2, demonstrating the optimal TR for each concentration of Gd-DTPA for a range of TE values. Higher concentrations of gadolinium greatly reduce optimal TR values, however after 15mM the SNR efficiency begins to drop for TE above 20ms. Given a minimum TE dictated by other factors such as matrix size or b-value. Figure 2 can be used to determine how much Gd-DTPA with which TR will give the greatest SNR efficiency. Table 1 summarizes the improvements in SNR of our proposed protocol using 2% PFA, an optimal concentration of gadolinium, and rehydration for 5 weeks, with an example TE of 26ms.
Conclusion
Rat brain tissue preparation can be tailored for MRI by altering fixative concentration, using an optimal concentration of gadolinium, and rehydrating the sample after fixation. An optimal tissue preparation results in improvements in SNR efficiency, allowing more possibilities to study tissue microstructure at high resolution with ex vivo MRI.Acknowledgements
Thanks to funding from the Wellcome Trust, and assistance and support from the BRAIN Centre for Preclinical Imaging, and the members of the Natbrainlab.References
1. Shepherd TM, Flint JJ, Thelwall PE, Stanisz GJ, Mareci TH, Yachnis AT, Blackband SJ. Postmortem interval alters the water relaxation and diffusion properties of rat nervous tissue — Implications for MRI studies of human autopsy samples. Neuroimage 2009;44:820–826. doi: 10.1016/j.neuroimage.2008.09.054.
2. D’Arceuil HE, Westmoreland S, de Crespigny AJ. An approach to high resolution diffusion tensor imaging in fixed primate brain. Neuroimage 2007;35:553–565. doi: 10.1016/j.neuroimage.2006.12.028.
3. Birkl C, Soellradl M, Toeglhofer AM, et al. Effects of concentration and vendor specific composition of formalin on postmortem MRI of the human brain. Magn. Reson. Med. 2017;44:820. doi: 10.1002/mrm.26699.
4. Hoffman EA, Frey BL, Smith LM, Auble DT. Formaldehyde Crosslinking: A Tool for the Study of Chromatin Complexes. J. Biol. Chem. 2015;290:26404–26411. doi: 10.1074/jbc.R115.651679.
5. Sutherland BW, Toews J, Kast J. Utility of formaldehyde cross-linking and mass spectrometry in the study of protein-protein interactions. J Mass Spectrom 2008;43:699–715. doi: 10.1002/jms.1415.
6. Thelwall PE, Shepherd TM, Stanisz GJ, Blackband SJ. Effects of temperature and aldehyde fixation on tissue water diffusion properties, studied in an erythrocyte ghost tissue model. Magn. Reson. Med. 2006;56:282–289. doi: 10.1002/mrm.20962.
7. Shepherd TM, Thelwall PE, Stanisz GJ, Blackband SJ. Aldehyde fixative solutions alter the water relaxation and diffusion properties of nervous tissue. Magn. Reson. Med. 2009;62:26–34. doi: 10.1002/mrm.21977.
8. Johnson GA, Calabrese E, Badea A, Paxinos G, Watson C. A multidimensional magnetic resonance histology atlas of the Wistar rat brain. Neuroimage 2012;62:1848–1856. doi: 10.1016/j.neuroimage.2012.05.041.
9. Calabrese E, Badea A, Watson C, Johnson GA. A quantitative magnetic resonance histology atlas of postnatal rat brain development with regional estimates of growth and variability. Neuroimage 2013;71:196–206. doi: 10.1016/j.neuroimage.2013.01.017.
10. Cahill LS, Laliberté CL, Ellegood J, Spring S, Gleave JA, van Eede MC, Lerch JP, Henkelman RM. Preparation of fixed mouse brains for MRI. Neuroimage 2012;60:933–939. doi: 10.1016/j.neuroimage.2012.01.100.
Figures