3229
Free watER iNvariant Estimation of Tensor (FERNET): Addressing the Issue of Edema in Clinically Feasible Acquisitions1Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Synaptive Medical Inc., Toronto, ON, Canada, 3IRISA, CNRS, Rennes, France, 4Psychiatry and Radiology, Harvard Medical School, Boston, MA, United States
Synopsis
Despite the growing research in free water elimination (FWE) methods with advanced diffusion acquisition protocols, the need for robust single-shell based FWE remains, as this is the standard acquisition protocol in the clinic. This is especially important in the characterization of peritumoral regions with infiltration. However, single-shell FWE is an ill-posed problem, dependent on parameter initialization, solutions to which often fail to obtain a balanced correction between healthy and abnormal tissue. We introduce FERNET, a robust FWE protocol for single-shell data with a comprehensive investigation of initialization parameters based on a software simulated phantom where the ground truth is known.
Purpose
The purpose of this work is to design a robust single-shell dMRI-based FWE paradigm applicable to clinical acquisitions, with a comprehensive investigation of parameters, that helps to consistently separate a meaningful FW component representing the edema from the underlying tissue, represented by the corrected tensor. This will enable better edema invariant tractography for surgical planning, and generate measures of infiltration in peritumoral tissue, aiding treatment. Despite the ill-posed nature of this problem in single-shell data, previous solutions tried to eliminate FW contamination from edema1. However, due to the heuristic choice of initializing parameters, FW may have been under/over estimated in several regions of the brain dependent on the acquisition, leading to incorrect tracking and erroneous inferences about underlying tissue microstructure. In this work, we present FERNET, a comprehensive FWE paradigm, which addresses the initialization of the FW map and constrains the output solutions using choices of parameters suggested by simulated phantoms.Methods
Data acquisition and preprocessing: T1 and dMRI data of 10 tumor (glioblastoma multiforme and metastasis) patients were selected (dMRI at TR/TE=5000/86ms, b=1000s/mm2, 30 gradient directions). T1 data was segmented using FSL-FAST to investigate tissue classes2.
FERNET: is a robust FWE method for clinically feasible diffusion acquisitions. This fits a two-compartment model per voxel1:
$$A_{i}(D,f)=fexp(-bq_i^T Dq_{i})+(1-f)exp(-bd)[1]$$
where the first term is the signal attenuation of the tissue compartment, modeled by a diffusion tensor, D; the second is the FW component; Ai is the signal attenuation of the ith diffusion weighted image; f is the contribution of the tissue compartment; qi is the ith gradient direction; b is the diffusion weighting; and d is the diffusivity (fixed at 3.0x10-3mm2/s) in the FW compartment. The bi-tensor model in [1] is an inverse problem with infinitely many solutions1, that we solve using a gradient-descent optimization after calculating an initial estimate of the solution. The initial estimate of attenuation in the tissue compartment $$$\hat{A}_{t}$$$ is crucial, but challenging for two reasons:
I- $$$\hat{A}_{t}$$$ is initialized based on estimating the contribution f to the diffusion signal per voxel, driven by an estimate of the representative unweighted signal in tissue and FW1 :
$$f (initial)= 1-\frac{log(\frac{S_{0}}{S_{t}})}{log(\frac{S_{w}}{S_{t}})} [2]$$
where S0, St, Sw correspond to the b0 signal, a representative b0 signal from the tissue compartment without free water, and a representative signal from pure FW compartment, respectively. Therefore, careful region-of-interest definition to obtain representative signals for each compartment is key for accurate FW estimation, leading to optimized estimation of tissue tensor D.
II- $$$\hat{A}_{t}$$$ is constrained by maximum (λmax) and minimum (λmin), the range of biologically plausible diffusivities in the tissue compartment1:
$$e^{-b\lambda_{max}}<\hat{A}_{t}<e^{-b\lambda_{min}}[3]$$
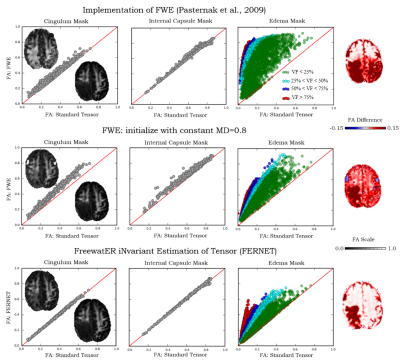
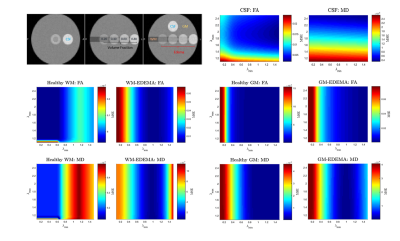
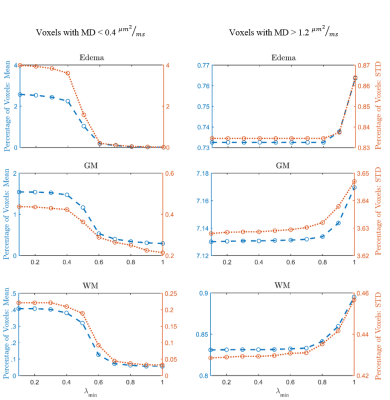
For (I), we estimate pure tissue signal as 95th percentile of b0 signal in a white matter (WM) mask (voxels with FA>0.70) and pure FW signal as the 95th percentile of voxels with high mean diffusivity (MD>2.8x10-3mm2/s). Initial restricted estimate of f by [3] prevents over-correction in healthy WM voxels. We verified that FERNET corrects only in edema regions with FW and not in tissue where FW is very low (contralateral cingulum and internal-capsule (IC)), by comparing FA values in these regions pre- and post-FERNET. These comparisons were repeated with previous single-shell FWE1 and its extension based on initializing with MD=0.8x10-3mm2/s. For (II), λmax and λmin were investigated using Phantomas, a software for simulating diffusion phantoms3. These parameter choices were based on mean square error (MSE) between FERNET output and ground truth values obtained by fitting a tensor to the same phantom, but without simulated edema (Fig.2). Due to the absence of ground truth in human data, we investigated voxels with MD outside a physiologically plausible range (0.4-1.2x10-3mm2/s) in WM, GM, and edema4.
Results and Discussion
FERNET FA maps in regions where FW is very low (cingulum, IC) were more consistent with the standard tensor fit FA values (Fig.1) when compared to previously proposed methods1. Additionally, FA values in FERNET corrected edema voxels were in a physiologically plausible range, compared to the abnormally high (~0.9) FA. FERNET of phantom data with varying parameters (Fig.2) shows that artificially high FA values in previous reports are likely due to erroneous initializing parameters. Phantom experiments suggest λmin=0.6 and λmax=2.5. Results from tumor data also show effective correction in edema using these parameters (Fig.3).Conclusion
We have proposed a FWE paradigm, FERNET, which addresses the known parameter dependence issues of single shell FWE methods, provides a robust selection of initialization parameters, and produces physiologically plausible solutions for the multi-compartment fit. As it separates the edema from the underlying tissue, this is a clinically viable paradigm for FWE that can be applied to clinical studies that have single-shell data and no means for advanced acquisition.Acknowledgements
This research was supported by the National Institutes of Health (NIH) grant 1R01NS096606 (PI: Ragini Verma), and research grant from Synaptive Medical 30071788 (PI: Ragini Verma).References
1- Pasternak, O., et al., Free water elimination and mapping from diffusion MRI. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine, 2009. 62: p. 717-30.
2- Zhang, Y., M. Brady, and S. Smith, Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Transactions on Medical Imaging, 2001. 20(1): p. 45-57.
3- Caruyer, E., et al. Phantomas: a flexible software library to simulate diffusion MR phantoms. in ISMRM. 2014.
4- Helenius, J., et al., Diffusion-Weighted MR Imaging in Normal Human Brains in Various Age Groups. AJNR Am J Neuroradiol, 2002. 23(2): p. 194-199.
Figures