3223
Developing 3D perfusion bioreactor for MRI and optical imaging1NIST, Boulder, CO, United States, 2Department of Mechanical Engineering, University of Colorado Boulder, Boulder, CO, United States, 3University of Colorado Boulder, Boulder, CO, United States
Synopsis
Whole-body medical imaging (such as MRI) can map many physical tissue parameters; however, there are currently many questions in the field regarding how changes in MRI are representative of changes in the underlying cells. To better understand these processes, we need to correlate MRI measurements with changes in microstructure. We created a living phantom for evaluation of techniques such as diffusion tensor imaging (DTI) that can be monitored and validated by optical techniques. Our future plan is to use MRI to study cell growth and monitor response to chemical and mechanical stimuli.
Introduction
Whole-body medical imaging (such as MRI) can map many physical tissue parameters on a length scale smaller than 1 mm1. However, in some cases, we have no clear understanding of how cellular processes and structure correlate with MRI signals and biomarkers2. Novel technologies are needed that can monitor cells, their differentiation, and their interactions non-invasively. Coupling of whole-body medical imaging measurements with microscopy techniques may allow radiologists to better understand the nature of observed lesions without the need for biopsy and pathologists to provide better guidance on observable signatures of different tissue abnormalities. Well-designed, in vitro, three-dimensional (3D) tissue cultures would allow us to study cell development and complex cell interactions, while controlling their environment. The purpose of this study was to develop a 3D MR compatible bioreactor for monitoring cell growth and differentiation via both MR and optical imaging.Materials and methods
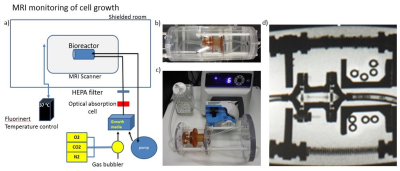
This study presents a simple, effective, gentle and easy-to-clean 3D bioreactor that enables perfusion of the hydro-gel containing cartilage cells as a model system with media, gasses bioactive agents, and contrast agents, which can be used in an incubator as well as in an MRI system. The bioreactor is composed from four major parts: perfusion chamber, tubing, optical absorption monitor, and peristaltic pump. In the bore of the magnet, the temperature is maintained by a heating jacket surrounding the phantom. Outside of the MRI, the phantom can be also used for cultivation of the cells in an incubator. To achieve functional and biomechanical stability of cells grown in an MRI system, several challenges had to be addressed. The first challenge was to develop 3D perfusion chamber suitable for both MRI and optical imaging. The second challenge was to determine the cell type and scaffolding to seed a 3D matrix with MRI-relevant dimensions. We tested three prototype designs (Figure 1), printed on a Formlabs printer using biocompatible resin Dental SG. Suitability of this material was proven by a 7 day culture of chondrocytes in a dish made of the resin to confirm no cytotoxicity. Whole volume of the gel was completely perfused through a channel in the central part of the gel as confirmed by Figure 2. The chondrocytes were seeded and routinely maintained in a 1% functionalized hyaluronic acid/ PEG-diacrylate hydrogel according to a protocol established by our lab3,4,5 (Figure 3). MRI images were acquired in 3T pre-clinical MRI scanner using GRE sequence, TR=500 ms, TE= 6.17 ms, 512x512 matrix; orientation coronal; flip angle 20°(Figure 4).Results
We built a non-magnetic bioreactor contained within a temperature jacket (allowing us maintain cells at 37 °C), which can be placed in the bore of the magnet in the shielded room. This bioreactor is connected to a perfusion apparatus and temperature control system outside of the shielded room. The perfusion apparatus provides continuous inflow of oxygenated culturing media (air with 5% CO2) and outflow of metabolite-containing deoxygenated media (Figure 5). The third prototype is the most suitable for further experiments, since it provides direct optical access for further cellular characterization under a confocal microscope. In addition, it is easy to fill, manipulate, and the design minimizes the risk of microbial infection.Discussion
The goal of this study was to fabricate a non-magnetic MRI-compatible perfused bioreactor to grow large 3D cell cultures that could be studied with quantitative MRI techniques such as DTI and MRS. We have incorporated optical access for standard microscopy and an optical absorption system for real time monitoring of in and output flows. The initial use of the bioreactor is to study growth and differentiation of artificial cartilage. The system goes beyond what is commercially available and allows automated physiological monitoring during growth, delivery of agents, application of mechanical stresses and imaging agents.Conclusion
We created a novel living phantom for evaluation of techniques such as diffusion tensor imaging (DTI). 3D cell scaffolds within a non-magnetic bioreactor provides a valuable research tool not only for DTI validation, but also to model disease processes and treatment. It provides the ability to use MR to detect, track, and quantify standardized, well-characterized tissue in vitro.Acknowledgements
No acknowledgement found.References
1. Alexander DC, Zikic D, Ghosh A, Tanno R, Wottschel V, Zhang J, Kaden E, Dyrby, Stamatios N, Sotiropoulos T B, Zhang H, Criminisi A. Image quality transfer and applications in diffusion MRI. NeuroI. 2017; 152: 283-298.
2. Jung-Hoon P, Sun W, and Cui M. High-resolution in vivo imaging of mouse brain through the intact skull. Proc Nat Ac Scie. 2015; 112(30): 9236-9241.
3. Unterman SA, Gibson M, Lee JH, Crist J, Chansakul T, Yang EC, and Elisseeff JH. Hyaluronic Acid-Binding Scaffold for Articular Cartilage Repair Tissue Eng Part A. 2012; 18(23-24): 2497–2506.
4. Novak T, Fites GK, Xu X, Worke L, Ciesielski A, Breur G, and Neu CP. Tis Eng Part A. 22(21-22): 1274-1285.
5. Brown BN and Badylak SF. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl Res. 2014; 163(4):268-285.
6. Youn I, Choi JB, Cao L, Setton LA, and Guilak F. Zonal variations in the three-dimensional morphology of the chondron measured in situ using confocal microscopy. Osteoart Cart, 2006; 14(9): 889-897.
Figures
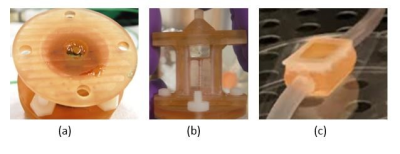
Figure 1. 3D perfusion bioreactors of different volumes tested for MRI a) The first prototype filled with a gel, b) the second prototype filled with chondrocytes in the gel and c) the third prototype with chondrocytes in the gel. Inner volumes of the chambers are (a) 1 ml (diameter = 1 cm, height=2 cm, width=1 cm), (b) 2.5 ml (depth=1 cm, width=1 cm, height=2.5 cm) and (c) 2 ml (depth=1 cm, width=1.2 cm, height=1.6 cm). Depth is the distance from the optical window on one side of the chamber to the optical window on the other side.
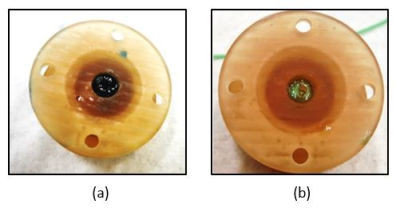
Figure 2. First prototype of the bioreactor a) perfused with media containing food coloring; b) reperfused with clear media to remove the color. This experiment confirmed full perfusion of the gel. The next step is to validate the perfusion of the third bioreactor.
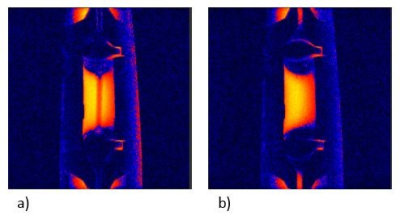
Figure 4. Cross-sectional magnetic resonance image from first prototype of the bioreactor. a) Perfused bioreactor; b) Unperfused bioreactor.
When the bioreactor is perfused with media, we can easily distinguish the channel that goes through the central part of the hydrogel in the bioreactor. With perfusion off, the channel disappears; there is no contrast between hydrogel and media filled channel. The scanning protocol: GRE sequence, TR=500 ms, TE= 4.85 ms, 256x256 matrix, orientation: coronal, flip angle: 20°.