3207
METastasis Reporting and Data System for Prostate Cancer (MET-RADS-P) for castration-resistant prostate cancer: prediction of clinical course, and identification of oligo-progressive lesions as targets for loco-regional ablative therapy.1Urology, Tokyo Medical and Dental University Graduate School, Tokyo, Japan, 2Biomedical Engineering, Tokai University School of Engineering, Kanagawa, Japan, 3Radiology, AIC Yaesu Clinic, Tokyo, Japan, 4Radiology, UMC Groningen, Groningen, Netherlands, 5Radiation Therapeutics and Oncology, Tokyo Medical and Dental University Graduate School, Tokyo, Japan
Synopsis
Whole-body diffusion-weighted MRI is a new-generation imaging tool for detecting prostate cancer. The extent of bone metastasis and the presence of visceral metastasis on whole-body diffusion-weighted MRI according to METastasis Reporting and Data System for Prostate Cancer (MET-RADS-P) were associated with a lower cancer-specific survival in castration-resistant prostate cancer. Furthermore, whole-body diffusion-weighted MRI facilitates identification of oligo-progressive lesions, which can be targets for loco-regional radiotherapy. MET-RADS-P score of whole-body diffusion-weighted MRI can be an imaging biomarker for castration-resistant prostate cancer in predicting clinical course, and identifying oligo-progressive lesions as targets for loco-regional ablative therapy.
Introduction
Whole-body diffusion-weighted MRI (WB-DWI) is a new generation imaging technique for prostate cancer1,2. Recently, METastasis Reporting and Data System for Prostate Cancer (MET-RADS-P) has been proposed as a standard of data acquisition, interpretation, and reporting for WB-MRI, including WB-DWI performed in men with advanced prostate cancer3. However, the clinical significance of the scores in castration-resistant prostate cancer (CRPC) has not been demonstrated. Loco-regional ablative treatment of oligometastatic disease has generated interest in many types of malignancies. Although the survival benefit of loco-regional treatment, in addition to standard care, for oligo-progressive CRPC, has not fully been demonstrated so far, WB-DWI is of increasing importance to diagnose oligo-progressive disease because of its better accuracy for detecting osseous metastasis than the combination of CT and bone scan, and WB-MRI even allows to assess the therapeutic response4,5. Here, we analyzed the prognostic impact of the MET-RADS-P scores of WB-DWI in CRPC patients, and the treatment outcomes of loco-regional radiotherapy for oligo-progressive lesions on WB-DWI.Materials & Methods
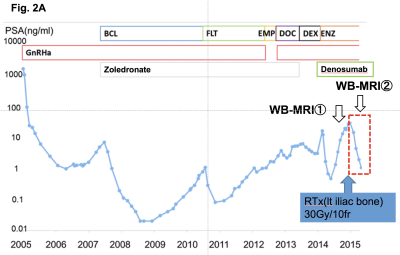
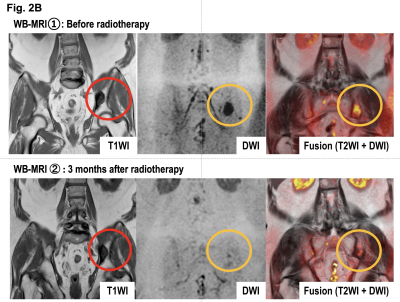
We retrospectively evaluated WB-MRI including WB-DWI obtained from 55 CRPC patients between 2014 and 2016, at the time of starting a new line of anticancer therapy. Twenty-two (39%) and 28 (49%) patients had a treatment history that included taxane-based chemotherapy and new hormonal drugs, respectively. A single radiologist reviewed WB-MRI for the number of bone metastases as well as for the presence of lymph node and visceral metastases according to the MET-RADS-P criteria. The relationship of the MET-RADS-P scores and clinical variables with cancer-specific survival (CSS) from WB-DWI examination was evaluated. For oligo-progressive CRPC with fewer than three progressive lesions, loco-regional radiotherapy and unchanged continuation of systemic therapy were recommended. Radiotherapy of 60-78 Gy to the prostate/lymph nodes metastasis or 30 Gy to the bone metastasis was applied. The PSA response to the loco-regional radiotherapy and the time to PSA progression were analyzed.Results
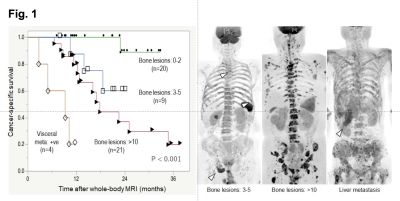
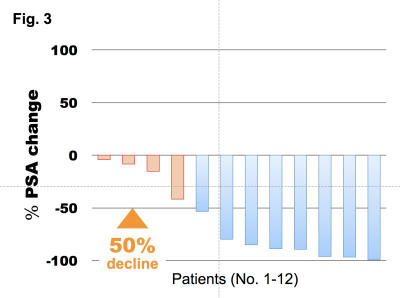
The median PSA level of eligible patients was 25 ng/ml (range: 0.22 ‒ 1428 ng/ml). Bone metastases were identified on WB-DWI in 49 patients (97%; number of bone metastasis = 0/1-2/3-5/6-10/>10: n = 8/15/9/0/25, respectively). Six (10%) and 5 (9%) patients had lymph node and visceral metastases, respectively. During the median follow-up period of 14 months (2.8 ‒ 38 months), 20 (35%) died of prostate cancer. Of the clinical variables and MET-RADS-P scores, high osseous metastatic burden (n = 3 - 5, and >10) and presence of visceral metastasis were significant independent indicators of shorter CSS (P = 0.03, < 0.001, 0.001, respectively, Figure). The 1-year CSS of the patients with bone metastasis (n = 0-2/3-5/>10) without visceral metastasis was 100, 88, and 80%, respectively, and that of the patients with visceral metastasis was 20%. Fifteen (29%) patients were diagnosed as having oligo-progressive CRPC. The most frequent sites for oligo-metastasis were the prostate (n = 7) and pelvic bone (n = 7), followed by the thoracic (n = 3) and lumbosacral (n = 2) spine. Of these oligo-progressive CRPC cases, 12 patients underwent loco-regional radiotherapy. The targets for the regional radiotherapy were the prostate/pelvic lymph nodes (n = 3), bone (n = 8), or both (n = 1). A decline in PSA levels of at least 50% in response to the radiotherapy was observed in 8 (67%) patients; the median time to PSA progression was 4.5 months (range, 1 – 9 months).Discussion
We showed here the usefulness of MET-RADS-P scoring for CRPC in predicting clinical course and identifying oligo-progressive lesions as targets for loco-regional ablative therapy. Given the advantage of WB-DWI in assessing tumor activity and therapeutic response in bone metastasis, which is not measurable by the RECIST guidelines, MET-RADS-P scoring of WB-DWI is a promising tool to help clinical-decision making for CRPC. Although the clinical utility and the reproducibility of MET-RADS-P needs to be validated in further works, MET-RADS-P will likely be a standard for the interpretation of WB-MRI.Conclusion
This is the first study to show the utility of MET-RADS-P scoring for CRPC in predicting clinical course and identifying targets for loco-regional ablative therapy.Acknowledgements
NoneReferences
1. Takahara T, Imai Y, Yamashita T, et al. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med. 2004;22(4):275-82.
2. Gillessen S, Attard G, Beer TM, et al. Management of Patients with Advanced Prostate Cancer: The Report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur Urol. 2017 Jun 24. pii: S0302-2838(17)30497-9.
3. Padhani AR, Lecouvet FE, Tunariu N, et al. METastasis Reporting and Data System for Prostate Cancer: Practical Guidelines for Acquisition, Interpretation, and Reporting of Whole-body Magnetic Resonance Imaging-based Evaluations of Multiorgan Involvement in Advanced Prostate Cancer. Eur Urol. 2017;71(1):81-92.
4. Lecouvet FE, Talbot JN, Messiou, et al. Monitoring the response of bone metastases to treatment with Magnetic Resonance Imaging and nuclear medicine techniques: a review and position statement by the European Organisation for Research and Treatment of Cancer imaging group. Eur J Cancer. 2014;50(15):2519-2531.
5. Lecouvet FE, El Mouedden J, Collette L, et al. Can whole-body magnetic resonance imaging with diffusion-weighted imaging replace Tc 99m bone scanning and computed tomography for single-step detection of metastases in patients with high-risk prostate cancer? Eur Urol. 2012;62(1):68-75.
Figures