3203
Assessment of Liver Fibrosis: Comparison of Diffusion Kurtosis Imaging, Conventional DWI, Aspartate Aminotransferase-to-Platelet Ratio Index and Fibrosis-41Zhongshan Hospital, Fudan University, Shanghai, China, 2MR Application Predevelopment, Siemens Healthcare, Erlangen, Germany, 3Siemens Shenzhen Magnetic Resonance Ltd, Shenzhen, China, 4MR Collaboration NE Asia, Siemens Healthcare, Shanghai, China
Synopsis
Diffusion kurtosis imaging (DKI) is a recently developed diffusion model that measures the non-Gaussian diffusion of water molecules in biological tissue. Few studies reported the potential of DKI on assessing hepatic fibrosis. Aspartate aminotransferase-to-platelet ratio index (APRI) and fibrosis-4 (FIB-4) are widely used non-invasive serum tests that estimate liver fibrosis. We compared the diagnostic performance of DKI, conventional DWI, APRI, and FIB-4 for evaluating the severity of liver fibrosis. Our results showed that diffusion-based measurements offer a similar diagnostic performance to the serum fibrosis biomarkers APRI and FIB-4 index for predicting liver fibrosis in patients with chronic liver disease.
INTRODUCTION
Aspartate aminotransferase-to-platelet ratio index (APRI) and fibrosis-4 (FIB-4) are widely used non-invasive serum tests that estimate liver fibrosis. Previous studies on liver explant and animals exhibited that DKI had potential for assessing hepatic fibrosis1,2. The purpose of this study was to compare the diagnostic performance of diffusion kurtosis imaging (DKI), conventional DWI, and serum fibrosis biomarkers APRI and FIB-4 for evaluating the severity of liver fibrosis in patients with chronic liver disease.METHODS
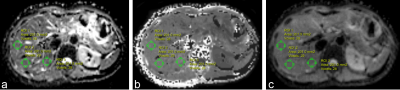
This prospective study was approved by the institutional review board, and written informed consent was obtained from each patient before participation. A total of 81 patients (59 men and 22 women; mean age, 57.6 ± 9.1 years; age range, 35 – 75 years) were included and underwent multi-b DWI with a 1.5T MR scanner (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany). The prototype multi-b DWI sequence was performed with b-values of 0, 200, 500, 1000, 1500, and 2000 sec/mm2, averages of 1, 1, 2, 2, 3, 4, respectively, repetition time/echo time of 8000 msec/63 msec, section thickness of 5 mm, gap of 1 mm, field of view of 380 × 308 mm2, and matrix of 128 × 128. DKI-derived mean diffusivity (MD), mean kurtosis (MK), and conventional apparent diffusion coefficient (ADC) maps were calculated and 2D round region of interests (Fig.1) were analyzed by using prototype software (Body Diffusion Toolbox; Siemens Healthcare, Erlangen, Germany) from the same multi-b DWI data. Liver function tests and platelet counts were performed, and the APRI and FIB-4 index were calculated using the corresponding formulas. The liver fibrosis stage was histologically determined based on the Scheuer scoring system: S0 (n = 16), S1 (n = 11), S2 (n = 13), S3 (n = 15), and S4 (n = 26). Interobserver repeatability of two observers on diffusion parameter measurements was assessed by using the intraclass correlation coefficient (ICC) with 95% confidence interval (CI). The correlation between the MD, MK, ADC, APRI, and FIB-4 index with fibrosis stage was assessed by Spearman's rank correlation coefficients. The area under receiver operating characteristic (AUROC) curves for the MD, MK, ADC, APRI, and FIB-4 index were compared to assess their diagnostic value in identifying liver fibrosis.RESULTS
Interobserver agreements of MD, MK and ADC between the two observers were excellent (ICC=0.836−0.848, 95 % CI: 0.763−0.899). The distribution of the diffusion-based measurements (MD, MK, and ADC) and the serum fibrosis biomarkers APRI and FIB-4 index according to liver fibrosis stages are shown in Fig.2. The MD, MK, ADC, APRI, and FIB-4 index showed significant correlation with the liver fibrosis stage (rho: -0.491, 0.537, -0.496, 0.485, and 0.387, respectively, P < 0.001). The AUROC curves of the MD, MK, ADC, APRI, and FIB-4 index for the diagnosis of significant disease (S ≥ 2), advanced fibrosis (S ≥ 3), and cirrhosis (S=4) are shown in Fig 3. The area under the curve (AUCs) values for predicting significant fibrosis, advanced fibrosis, and cirrhosis were not significantly different (P > 0.05) between the diffusion-based measurements (MD, MK, and ADC) and the serum fibrosis biomarkers, APRI and FIB-4 index.
CONCLUSION
Diffusion-based measurements including DKI and conventional DWI offer similar diagnostic performance to the serum fibrosis biomarkers APRI and FIB-4 index for predicting liver fibrosis in patients with chronic liver disease.Acknowledgements
No acknowledgement found.References
1. Rosenkrantz AB, Sigmund EE, Winnick A et al (2012) Assessment of hepatocellular carcinoma using apparent diffusion coefficient and diffusion kurtosis indices: preliminary experience in fresh liver explants. Magn Reson Imaging 30:1534-1540
2. Anderson SW, Barry B, Soto J, Ozonoff A, O'Brien M, Jara H (2014) Characterizing non-gaussian, high b-value diffusion in liver fibrosis: Stretched exponential and diffusional kurtosis modeling. J Magn Reson Imaging 39:827-834
Figures