3200
Predictive and prognostic value of intravoxel incoherent motion (IVIM) MR imaging in patients with advanced cervical cancers undergoing concurrent chemo-radiotherapy1Department of Radiology, Drum Tower Hospital, School of Medicine, Nanjing University, Nanjing, China, 2Philips Healthcare, Shanghai, Shanghai, China
Synopsis
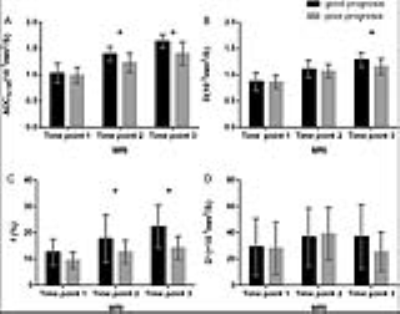
Pelvic IVIM MR imaging were performed on 30 women with advanced cervical cancers at three time points. The performance of tumour size and IVIM-derived parameters in predicting long-term prognosis was evaluated. After a median follow-up of 24 months, 83.33% patients were alive, 70.00% remained free of disease. A shrinkage rate of maximum diameter≥ 58.31% was useful in predicting a good long-term prognosis. The IVIM-derived ADCIVIM value at time point 2 and the ADCIVIM and f values at time point 3 also performed well in predicting a good prognosis. IVIM has great potential in predicting long-term prognosis in patients with advanced cervical.
INTRODUCTION
By using the intravoxel incoherent motion (IVIM) model, the diffusion-related coefficient (D) and the perfusion-related parameter (f) can be obtained simultaneously. Here, we explored the application of IVIM MR imaging in predicting long-term prognosis in patients with advanced cervical cancers treated with concurrent chemo-radiotherapy (CCRT).METHODS
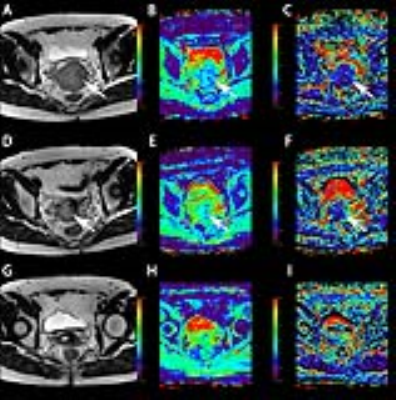
Pelvic MR examinations including IVIM sequence were performed in 30 women with advanced cervical cancers at three time points (within 2 weeks before, 2 and 4 weeks after the initiation of CCRT). The performance of tumor size and IVIM-derived parameters in predicting longterm prognosis was evaluated.RESULTS
After a median follow-up of 24 months (range, 10∼34 months), 25/30 (83.33%) patients were alive, and 21/30 (70.00%) remained free of disease. A shrinkage rate of maximum diameter (time point 1 vs. 3) ≥ 58.31% was useful in predicting a good long-term prognosis. The IVIM-derived apparent diffusion coefficient (ADCIVIM) value at time point 2 and the ADCIVIM and f values at time point 3 also performed well in predicting a good prognosis, with AUC of 0.767, 0.857 and 0.820, respectively.DISCUSSION
Predictive value of short-term outcomes evaluated with RECIST. The long-term prognosis was quite different from the short-term outcome evaluated with RECIST, with a sensitivity of 71.43% and a specificity of 44.44% in our study , which similar with Vincens et al’s findings.1 Therefore, the short-term outcome evaluated with RECIST had a high false positive rate and a moderate false negative rate in predicting long-term outcomes for patients with cervical cancers treated with CCRT. This might because the radiation changes (inflammation, edema, and capillary hypervascularity) during the therapy may misleading the accurate differentiation between residual tumor and therapy induced changes.
Predictive ability of tumour size. After the therapy started, the tumour size of the cervical cancer continued to decrease, and the good responders showed a greater rate of decrease than the poor responders. At time point 3 , an atrophy rate of maximum diameter over 58.31% proved effective in predicting a good long-term prognosis with an AUC of 0.751. Mayr et al. have reported similar results, in that tumours with fast regression rate during mid-therapy have the best rates of local control and disease-free survival in patients with cervical cancers.2 However, evaluation of the tumour size was less effective and provided less rapid results than the IVIM parameters, as discussed below.
Predictive ability of the f value. The f value is known as the vascular volume fraction of the voxel. A lower f value might indicate lower blood supply and hypoxic status of the tumour tissues, which are involved in local treatment failure after radiation therapy, a high incidence of regional or distant metastases, and poor disease-free survival rates in multiple solid tumours, including cervical cancer.3 In addition, Christine et al. have reported that cervical cancers with low perfusion derived by DCE MR imaging are likely to be resistant to radiation treatment as well as to have a high probability of developing metastases4; these results may indirectly support our findings.
Predictive value of diffusion related parameters. The ADCIVIM, D and ADCDWI values of cervical cancer continued to increase during CCRT, possibly because of the decrease in cellular density and destruction of cellular membranes after effective treatment. At time points 2 and 3, a high ADCIVIM value of cervical cancer predicted a good prognosis, with an AUC of 0.767 and 0.857, respectively. Somoye et al. have reported similar results in a study of patients with cervical cancers, in which survivors had a higher mid-treatment value (1.55 × 10−3 mm2/s) than non-survivors (1.36 × 10−3 mm2/s).5 Moreover, in the same study, a mid-treatment ADC value less than 1.40 × 10−3 mm2/s has been found to be associated with adverse outcomes, similarly to our results (cut-off value of ADCIVIM at time point 2, 1.29 × 10−3 mm2/s). However, the pure diffusion coefficient D value demonstrated no significant power, thus indicating that perfusion effects might be an essential part of predicting treatment response. According to our results, the IVIM sequence provided more diversified as well as more powerful parameters in predicting the long-term prognosis of advanced cervical cancer at early stages of CCRT. The IVIM parameters allow for earlier and more accurate (AUC of 0.857) prediction of long-term prognosis. Furthermore, the IVIM parameters showed a significant positive relationship with different follow-up time points, but the age and FIGO stage of patients had weak associations with the parameters.
DISCUSSION
IVIM MR imaging has great potential in predicting long-term prognosis in patients with advanced cervical cancers treated with CCRT.Acknowledgements
No acknowledgement found.References
1. Vincens, E, Balleyguier, C, Rey, A, et al. Accuracy of magnetic resonance imaging in predicting residual disease in patients treated for stage IB2/II cervical carcinoma with chemoradiation therapy. Cancer 2008;113:2158-2165.
2. Eifel, PJ, Jhingran, A, Levenback, CF, et al. Predictive Value of a Proposed Subclassification of Stages I and II Cervical Cancer Based on Clinical Tumor Diameter. Int J Gynecol Cancer 2009;19:2-7.
3. Vaupel, P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol 2004;14:198-206.
4. Ellingsen, C, Hompland, T, Galappathi, K, et al. DCE-MRI of the hypoxic fraction, radioresponsiveness, and metastatic propensity of cervical carcinoma xenografts. Radiother Oncol 2014;110:335-341.
5. Somoye, G, Harry, V, Semple, S, et al. Early diffusion weighted magnetic resonance imaging can predict survival in women with locally advanced cancer of the cervix treated with combined chemo-radiation. Eur Radiol 2012;22:2319-2327.
Figures