3198
Diffusion kurtosis imaging in the characterization of rectal cancer: Evaluation of segmentation strategies and repeatability1Department of Radiology;Department of Oncology, Fudan University Shanghai Cancer Center; Shanghai Medical College, Fudan University, Shanghai, China, 2Fudan University Shanghai Cancer Center; Shanghai Medical College, Fudan University, Shanghai, China, 3MR Application Predevelopment, Siemens Healthcare, Erlangen, Germany, 4Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, China;, Shanghai, China, 5MR Collaboration NE Asia, Siemens Healthcare, Shanghai, China;, Shanghai, China
Synopsis
The aim of this study was to evaluate the influence of different segmentation strategies on diffusion parameters and the performance of diffusion kurtosis imaging in predicting rectal cancer histopathological characteristics before a treatment decision is made. The results show that the whole-tumor-volume segmentation strategy could achieve the best inter- and intra-observer repeatability among the six different strategies, and DKI with this segmentation strategy performed accurately for differentiating between well-differentiated and poorly to moderately differentiated patients.
Background and purpose
Previous studies have shown that the region of interest (ROI) size and positioning influence tumor apparent diffusion coefficient (ADC) values and inter-observer variability in malignant tumors [1-3]. However, there are few reports concerning the influence of segmentation strategy on DKI-derived parameter measurement. The robustness and repeatability of DKI measures in rectal cancer needs to be established, and this is particularly important for multicenter trials. The objective of this study was to assess the influence of segmentation strategies on multiple diffusion parameters, including DKI and conventional DWI, and to explore the most repeatable strategy for correlation of these diffusion parameters with rectal cancer characteristics.Methods
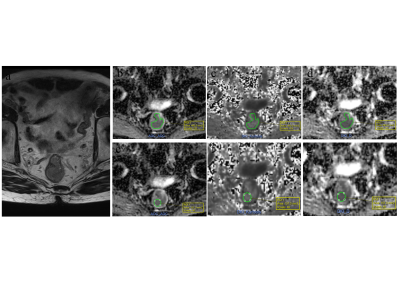
A total of 66 patients (41 male, 25 female) with rectal cancer were included in our study. Institutional review board approval was obtained, and all the patients signed informed consent. MRI was performed on a 3T MR scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) with a 16-channel phased-array body coil. An axial multi-b EPI DWI prototype sequence was performed with the following parameters: TR/TE = 4500/82 ms; FOV = 200 × 180 mm2; slice thickness = 6 mm; scan matrix = 140 × 140; voxel size = 1.4 × 1.4 × 6 mm3; phase oversampling = 20%; no. of slices = 20; tri-directional diffusion gradients were performed with b values of 0, 700, 1400 and 2100s/mm2 (with no. of averages = 1, 2, 4, and 6, respectively; GRAPPA acceleration factor = 2; and acquisition time = 3 min, 51 sec. Patients did not receive any bowel preparation before the MRI examination. The pixel-wise parameter maps of DKI (corrected diffusion coefficient, D; diffusion kurtosis coefficient, K) and conventional DWI (apparent diffusion coefficient, ADC) were both calculated from the same multi-b DWI data with all the measured b values by using the prototype post-processing software, Body Diffusion Toolbox (Siemens Healthcare, Erlangen, Germany). The histopathology factors, including the histological grade, T stage, N stage, extranodal tumor deposits, neural invasion, and peritumor-intravascular cancer emboli, were selected from the database. Two readers independently measured the parameters using six segmentation strategies, including three slice protocols (single slice with the largest tumor area, three slices with the largest tumor areas, and all tumor slices), combined with two ROI shapes (freehand tumor outline and circular shape) (Figure 1). Kruskal-Wallis, a paired-sample t-test, interclass correlation coefficient (ICC), Bland and Altman, Student's t-tests, and receiver operating characteristic curves were used for the statistical analysis.Results
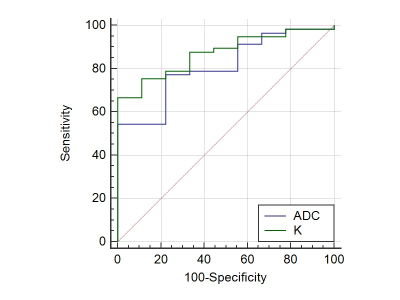
There was no significant difference in the ADCs derived from different slice protocols (p > 0.05) for either the tumor-outline or circular ROI. The same results were observed for the K and D values (p > 0.05). For all three slice protocols, the ADC and D values from the tumor-outline ROIs were significantly higher than those from the circular ones (p < 0.05), whereas the K values from the umor-outline ROI were significantly lower than those from the circular ROI (p < 0.05). The whole-tumor-volume ROI (all tumor slices + tumor-outline ROI) strategy achieved the highest ICC among the six strategies. By utilizing the whole-tumor-volume ROI, the K of the poorly to moderately- differentiated tumors (0.993 ± 0.105) was significantly higher than that of the well-differentiated tumors (0.905 ± 0.059) (p = 0.037). The D was 1.258 ± 0.232 vs. 1.165 ± 0.203, (p = 0.267), and the ADC was 0.664 ± 0.066 ×3 mm2/s vs. 0.721 ± 0.064 ×3 mm2/s (p = 0.034). The AUCs of K and ADC for the assessment of the well-differentiated tumors were 0.871 and 0.809, respectively. The optimal cutoff values of K and ADC for the best identification of patients with well-differentiated tumors were 0.959 (sensitivity, 100% and specificity, 66.67%) and 0.697 ×10-3 mm2/s (sensitivity, 77.78% and specificity, 77.19%), respectively (Figure 2).
Discussion and Conclusion
Our study showed that different segmentation strategies significantly influence the measurements of DKI and conventional DWI-derived parameters. A whole-tumor-volume approach could yield the most repeatable result among all six methods. The kurtosis-derived parameter from DKI performed well for differentiating between poorly -to moderately differentiated and well-differentiated tumors, compared with diffusivity and ADC, which is consistent with the results from Zhu et al [4]. Their results also showed that the K values are significantly higher in grade 3 (G3) vs. G1-2 rectal adenocarcinomas, and G3 is the poorest differentiated grade, according to the World Health Organization (WHO) grading criteria [4]. In conclusion, the K value of the DKI model obtained from the whole-tumor-volume may have considerable diagnostic value for the estimation of rectal cancer characteristics.Acknowledgements
No acknowledgement found.References
1. Lambregts DM, Beets GL, Maas M et al (2011) Tumour ADC measurements in rectal cancer: effect of ROI methods on ADC values and interobserver variability. Eur Radiol 21:2567-74
2. Chao M, Guo X, Li L et al (2017) Effect of region of interest size on ADC measurements in pancreatic adenocarcinoma Cancer Imaging 17:13
3. Colagrande S, Pasquinelli F, Mazzoni LN, Belli G, Virgili G (2010) MR-diffusion weighted imaging of healthy liver parenchyma: repeatability and reproducibility of apparent diffusion coefficient measurement. J Magn Reson Imaging JMRI 31:912-920
4. Zhu L, Pan Z, Ma Q et al (2017). Diffusion Kurtosis Imaging Study of Rectal Adenocarcinoma Associated with Histopathologic Prognostic Factors: Preliminary Findings. Radiology 284: 66-76
Figures