3197
IVIM DWI in the assessment of renal diffusion and perfusion alternations in ischemic acute kidney injury (AKI) animals1Institute for Medical Imaging Technology, Shanghai Jiao Tong university, Shanghai, China, 2Academy for Advanced Interdisciplinary Studies, Peking University, Beijing, China, 3College of Engineering, Peking University, Beijing, China, 4Philips Healthcare, Suzhou, China, 5Department of Radiology, Peking University First Hospital, Beijing, China
Synopsis
Intravoxel incoherent motion (IVIM) DWI is able to simultaneously detect diffusion and perfusion characteristics of renal tissue, which provides more sensitive measurement of renal function than serum creatinine. This study investigates the feasibility of using IVIM DWI to evaluate renal diffusion and perfusion changes in ischemic AKI animals. IVIM DWI was performed on rabbits prior to (24 hours before surgery) and after the surgery (1 hour, 3 hours, 1 week and 2 weeks after surgery). After injection of 0.8 mg microspheres, a noticeable change of renal diffusion and perfusion can be seen in the cortex immediately after the surgery. Pathological results also confirmed the renal injury with findings of ischemic and wrinkled features with dilated change of Bowman’s capsule.
Background
Ischemic acute kidney injury (AKI) is a prevalent cause of renal failure [1,2]. It is recognized that the occurrence of ischemic-AKI involves complex pathological changes including hemodynamic alternation, tissue hypoxia and inflammation [3]. Intravoxel incoherent motion (IVIM) DWI is able to simultaneously detect diffusion and perfusion characteristics of renal tissue, which provides more sensitive measurement of renal function than serum creatinine.Purpose
To investigate the feasibility of using IVIM DWI to evaluate renal diffusion and perfusion changes in ischemic AKI animals.Materials and Methods
Animal Model:
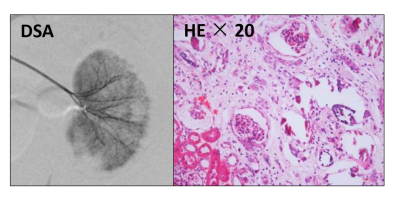
All procedures used in this study were approved by our local Research Ethics Committee. Six male New Zealand rabbits with body weights of 2.5-3.5 kg were included in the study. To establish ischemic AKI model, we injected 0.8 mg microspheres (acryl beads, 40 to 120 μm in diameter) into the ostium of the right renal artery under anesthesia condition. MRI scan was performed at different time to evaluate progressive changes of the AKI kidney. Four weeks after the surgery, pathological images of H&E staining were obtained.
MR Imaging:
IVIM DWI was performed on rabbits (n = 6) prior to (24 hours before surgery) and after the surgery (1 hour, 3 hours, 1 week and 2 weeks after surgery) using a 3.0 T MRI scanner (Achieva, Philips Medical Systems). During the MRI experiments, anesthesia was induced with isoflurane (1.0 L/min) delivered by a facemask. DWI was performed using a single-shot spin-echo echo-planar (EPI) sequence (coronal, TR = 3000 ms, TE = 65 ms, acquired matrix = 98 × 80, FOV = 150 × 150 mm2, slice thickness = 5 mm, gap = 1.0 mm, SNESE factor = 2). The following b-values were used for acquisition: 0, 20, 40, 60, 80, 100, 150, 200, 300, 400, 500, 600, 700, 800, 900 and 1000 sec/mm2. Respiratory triggering was applied to make the images motion insensitive. Quantitative Analysis: Before parameter fitting, a two-dimensional affine registration was performed to mitigate respiratory motion artifacts using an in-house software. According to the IVIM model, the DWI signal can be related as follows:
$$ S_b/S_0=(1-f)\cdot\exp(-bD)+f\cdot\exp(-bD*) $$
where S0 is the signal intensity at b = 0, Sb is the signal intensity at nonzero b value, D is the true diffusion coefficient, D* is the pseudodiffusion coefficient, and f is the fraction of microvascular volume. All the parameters were estimated using the least squares fitting algorithm. ADC was calculated using a monoexponential model with all b values. The changes in the diffusion (ADC, D) and perfusion parameters (D* and f) were analyzed in renal cortex, medulla and injuried renal region manually selected in T2w images.
Results
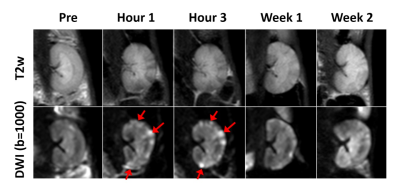
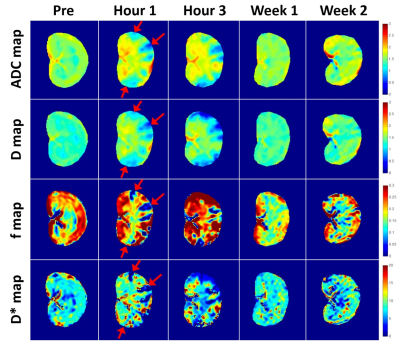
Fig 1 shows representative T2w and DWI images acquired before and after AKI surgery (1 hour, 3 hours, 1 week and 4 weeks after AKI surgery). Once in the bloodstream, micro-emboli lodged in small arteries and produced a microcrystalline angiitis, characterized by endothelial inflammatory reaction developing in a stepwise pattern (Fig 1, red arrows). Fig 2 shows the renal parametric maps of IVIM-metrics (ADC, D, D*, and f maps) of an AKI rabbit. The results show a clearly higher baseline diffusion rate (ADC) in healthy renal cortex (1.58 ± 0.03 ×10-3 mm2/s) than that in the medulla (1.45 ± 0.03 ×10-3 mm2/s), which is consistent with previous reports [4]. After injection of 0.8 mg microspheres, a noticeable change of renal diffusion and perfusion can be seen in the cortex immediately after the surgery (Fig 2, Hour 1, red arrows). Pathological results also confirmed the renal injury with findings of ischemic and wrinkled features with dilated change of Bowman’s capsule (Fig 3).
In the AKI cortex, D reduced progressively after surgery (Pre:1.34 ± 0.04 × 10-3 mm2/s; Hour 1: 0.95 ± 0.03 × 10-3 mm2/s; Hour 2: 0.93 ± 0.05 × 10-3 mm2/s) and increased starting after one week (Fig 4b). D* decreased to the bottom at the first hour after surgery (6.26 ± 2.18 ×10-3 mm2/s) and started to recover 3 hours after surgery (6.47 ± 2.37 × 10-3 mm2/s, Fig 4d). f fell from 0.24 ± 0.02 (Pre) to 0.04 ± 0.01 (Hour 1, P < 0.01), and returned 0.14 ± 0.02 after two weeks of recovery (Fig 4c).
Conclusions
Our study demonstrated the feasibility of using IVIM DWI to monitor the renal functional alternation in ischemic-AKI animals, implying that IVIM derived diffusion and perfusion parameters are promising biomarkers in the evaluation of ischemic renal injury. Compared to conventional DWI, IVIM model is capable of simultaneously assessing both diffusion and perfusion characteristics, thus is highly desired in the clinical evaluation of the progress of AKI.Acknowledgements
No acknowledgement found.References
1. Lameire N, Van Biesen W, Vanholder R. The changing epidemiology of acute renal failure. Nat Clin Pract Nephrol. 2006;2(7):364–377.
2. Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3(3):844–861.
3. Hörbelt M, Lee S Y, Mang H E, et al. Acute and chronic microvascular alterations in a mouse model of ischemic acute kidney injury. American Journal of Physiology Renal Physiology, 2007, 293(3):688-695.
4. Notohamiprodjo M, Chandarana H, Mikheev A, et al. Combined intravoxel incoherent motion and diffusion tensor imaging of renal diffusion and flow anisotropy. Magn Reson Med 2015, 73:1526–1532
Figures